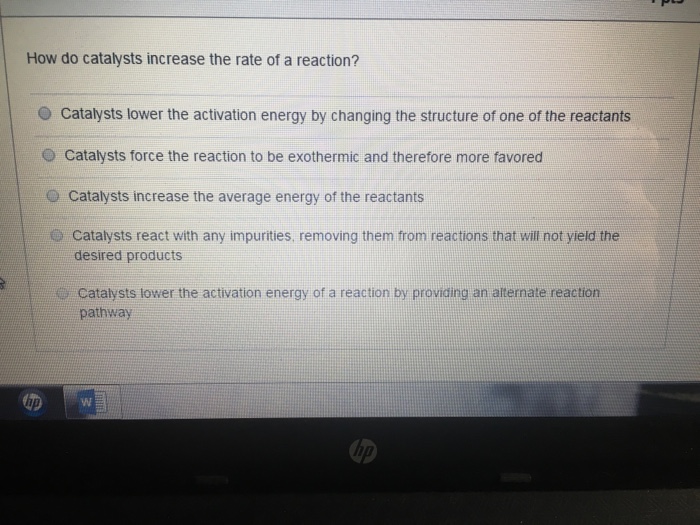
How Do Catalysts Increase The Rate Of A Reaction . Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Describe the similarities and differences between the. This page explains how adding a catalyst affects the rate of a reaction. It accomplishes this task by. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. However, not all reactions have suitable catalysts. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction.
from www.chegg.com
This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. This page explains how adding a catalyst affects the rate of a reaction.
Solved How do catalysts increase the rate of a reaction?
How Do Catalysts Increase The Rate Of A Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. It accomplishes this task by. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a.
From askfilo.com
How does a catalyst increase the rate of a reaction 3 Filo How Do Catalysts Increase The Rate Of A Reaction It accomplishes this task by. However, not all reactions have suitable catalysts. Describe the similarities and differences between the. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. What. How Do Catalysts Increase The Rate Of A Reaction.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change How Do Catalysts Increase The Rate Of A Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. This page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance that. How Do Catalysts Increase The Rate Of A Reaction.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID How Do Catalysts Increase The Rate Of A Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? This page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory. How Do Catalysts Increase The Rate Of A Reaction.
From sheetalschemblog.blogspot.com.tr
Sheetal's Chemistry Blog How Do Catalysts Increase The Rate Of A Reaction Describe the similarities and differences between the. This page explains how adding a catalyst affects the rate of a reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. It accomplishes this task by. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate. How Do Catalysts Increase The Rate Of A Reaction.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science How Do Catalysts Increase The Rate Of A Reaction However, not all reactions have suitable catalysts. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains. How Do Catalysts Increase The Rate Of A Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? How Do Catalysts Increase The Rate Of A Reaction What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. This page describes and explains the way that. How Do Catalysts Increase The Rate Of A Reaction.
From www.coursehero.com
[Solved] 4. What is a catalyst and how does it increase the reaction How Do Catalysts Increase The Rate Of A Reaction Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It accomplishes this task by. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by. How Do Catalysts Increase The Rate Of A Reaction.
From slideplayer.com
Lecture 1405 Reaction Mechanism and Catalysis ppt download How Do Catalysts Increase The Rate Of A Reaction The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. Catalysts work by. How Do Catalysts Increase The Rate Of A Reaction.
From slideplayer.com
Chapter 1 Rate of Reaction. ppt download How Do Catalysts Increase The Rate Of A Reaction This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the collision theory of reaction. However, not all reactions have suitable catalysts. A catalyst is a. How Do Catalysts Increase The Rate Of A Reaction.
From owlcation.com
Chemical Reactions and Chemical Equations Owlcation How Do Catalysts Increase The Rate Of A Reaction Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate. How Do Catalysts Increase The Rate Of A Reaction.
From spmchemistry.blog.onlinetuition.com.my
Factors Affecting the Rate of Reaction SPM Chemistry How Do Catalysts Increase The Rate Of A Reaction It accomplishes this task by. However, not all reactions have suitable catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to. How Do Catalysts Increase The Rate Of A Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson How Do Catalysts Increase The Rate Of A Reaction Describe the similarities and differences between the. What are catalysts, and how do they work in terms altering the parameters of a reaction? This page describes and explains the way that adding a catalyst affects the rate of a reaction. It accomplishes this task by. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate.. How Do Catalysts Increase The Rate Of A Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Do Catalysts Increase The Rate Of A Reaction A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. However, not all reactions have suitable catalysts. Describe the similarities and differences between the. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Revise how to measure rates of reaction and how to. How Do Catalysts Increase The Rate Of A Reaction.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent How Do Catalysts Increase The Rate Of A Reaction This page explains how adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Describe the similarities and differences between the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only. How Do Catalysts Increase The Rate Of A Reaction.
From slideplayer.com
Chapter 1 Rate of Reaction. ppt download How Do Catalysts Increase The Rate Of A Reaction This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Describe the similarities and differences between the. However, not all reactions have suitable catalysts. A catalyst is a substance that increases the rate of a chemical reaction without being used. How Do Catalysts Increase The Rate Of A Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse How Do Catalysts Increase The Rate Of A Reaction The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. This page describes and explains the way that adding. How Do Catalysts Increase The Rate Of A Reaction.
From www.slideshare.net
Rate of reaction(f5) How Do Catalysts Increase The Rate Of A Reaction The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? It accomplishes this task by. However, not all reactions have suitable catalysts. Revise. How Do Catalysts Increase The Rate Of A Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry How Do Catalysts Increase The Rate Of A Reaction Describe the similarities and differences between the. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction.. How Do Catalysts Increase The Rate Of A Reaction.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Do Catalysts Increase The Rate Of A Reaction A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. However, not all reactions have suitable catalysts. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration. How Do Catalysts Increase The Rate Of A Reaction.
From fyoefmgmv.blob.core.windows.net
Do Catalysts Increase Reaction Rate at Stephen Johnson blog How Do Catalysts Increase The Rate Of A Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction. It accomplishes this task by. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and. How Do Catalysts Increase The Rate Of A Reaction.
From www.numerade.com
SOLVEDHow does a catalyst increase the rate of a reaction? How Do Catalysts Increase The Rate Of A Reaction It accomplishes this task by. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. However, not all reactions have suitable catalysts. What are catalysts, and how do they work in terms altering. How Do Catalysts Increase The Rate Of A Reaction.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the How Do Catalysts Increase The Rate Of A Reaction However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? It accomplishes this task by. It assumes that you are already familiar with basic ideas about the collision theory of reaction.. How Do Catalysts Increase The Rate Of A Reaction.
From www.youtube.com
How do enzymatic catalysts increase the rates of reactions? YouTube How Do Catalysts Increase The Rate Of A Reaction Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a. How Do Catalysts Increase The Rate Of A Reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii How Do Catalysts Increase The Rate Of A Reaction What are catalysts, and how do they work in terms altering the parameters of a reaction? This page describes and explains the way that adding a catalyst affects the rate of a reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page explains how adding a catalyst affects the rate of a. How Do Catalysts Increase The Rate Of A Reaction.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 How Do Catalysts Increase The Rate Of A Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction. Describe the similarities and differences between the. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. It accomplishes. How Do Catalysts Increase The Rate Of A Reaction.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes How Do Catalysts Increase The Rate Of A Reaction It accomplishes this task by. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Describe the similarities and differences between the. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. Catalysts work by. How Do Catalysts Increase The Rate Of A Reaction.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii How Do Catalysts Increase The Rate Of A Reaction This page explains how adding a catalyst affects the rate of a reaction. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Describe the similarities and differences between the.. How Do Catalysts Increase The Rate Of A Reaction.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii How Do Catalysts Increase The Rate Of A Reaction Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst. How Do Catalysts Increase The Rate Of A Reaction.
From www.savemyexams.com
Measuring Rates Edexcel IGCSE Chemistry Revision Notes 2019 How Do Catalysts Increase The Rate Of A Reaction Describe the similarities and differences between the. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Catalysts work by attracting reactant molecules on to. How Do Catalysts Increase The Rate Of A Reaction.
From www.coursehero.com
[Solved] 4. What is a catalyst and how does it increase the reaction How Do Catalysts Increase The Rate Of A Reaction A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. It accomplishes this task by. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy.. How Do Catalysts Increase The Rate Of A Reaction.
From www.chegg.com
Solved How do catalysts increase the rate of a reaction? How Do Catalysts Increase The Rate Of A Reaction It assumes that you are already familiar with basic ideas about the collision theory of reaction. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. Only a very small mass of catalyst is needed to increase the rate of a reaction. It accomplishes this. How Do Catalysts Increase The Rate Of A Reaction.
From www.studypool.com
SOLUTION Rates of reaction catalysts mindmap Studypool How Do Catalysts Increase The Rate Of A Reaction It accomplishes this task by. However, not all reactions have suitable catalysts. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes that you are already familiar with basic ideas about the. How Do Catalysts Increase The Rate Of A Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Do Catalysts Increase The Rate Of A Reaction Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. However, not all reactions have suitable catalysts. Revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a. It assumes that you are already familiar with. How Do Catalysts Increase The Rate Of A Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Do Catalysts Increase The Rate Of A Reaction A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. It accomplishes this task by. Describe the similarities and differences between the. What are catalysts, and how. How Do Catalysts Increase The Rate Of A Reaction.
From www.gauthmath.com
Solved How does a catalyst increase the rate of a chemical reaction How Do Catalysts Increase The Rate Of A Reaction The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? However, not all reactions have suitable catalysts. Revise how to measure rates. How Do Catalysts Increase The Rate Of A Reaction.