Label Validation Definition . This paper begins a discussion on the varied ways to implement packaging validation. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance.
from www.orielstat.com
This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Effective process validation contributes significantly to assuring drug quality.
Medical Device Process Validation What You Need to Know
Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures.
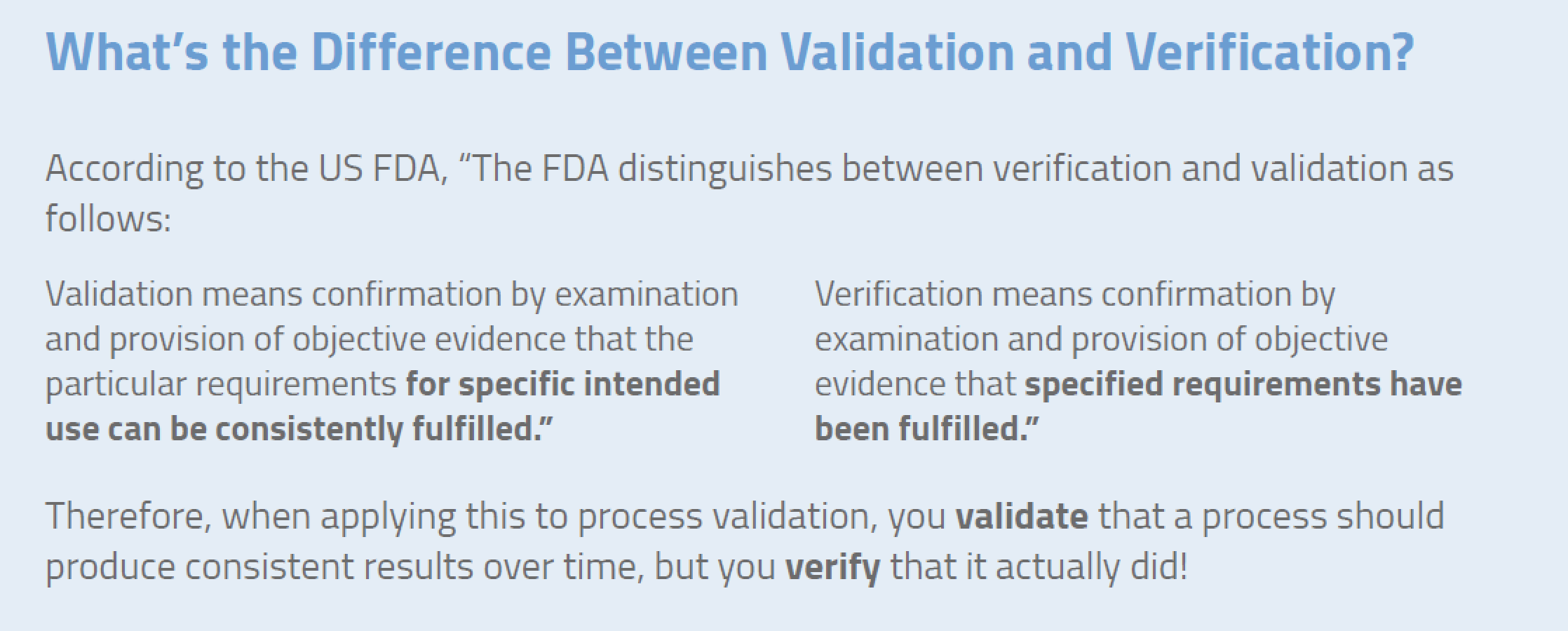
From qacraft.com
Validation Vs Verification Infographics QACraft Pvt. Ltd Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. (z) validation means confirmation by examination and. Label Validation Definition.
From www.testgorilla.com
A brief introduction to Test validation Label Validation Definition Effective process validation contributes significantly to assuring drug quality. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and. Label Validation Definition.
From www.slideserve.com
PPT Validation Part 5 Review and summary PowerPoint Presentation Label Validation Definition Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. The basic principle. Label Validation Definition.
From www.researchgate.net
(PDF) Definition and validation of the nursing diagnosis label “wish to Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. The basic principle of quality assurance. This paper begins a discussion on the varied ways to implement packaging validation. (z) validation means confirmation by examination and provision of objective. Label Validation Definition.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Label Validation Definition Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Effective process validation contributes significantly to assuring drug quality.. Label Validation Definition.
From www.slideserve.com
PPT Databases PowerPoint Presentation, free download ID2465214 Label Validation Definition Effective process validation contributes significantly to assuring drug quality. The basic principle of quality assurance. This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Structured product labeling (spl). Label Validation Definition.
From www.presentationeze.com
Product and Process Validation Process ValidationPresentationEZE Label Validation Definition This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. This guidance document describes the general labelling. Label Validation Definition.
From 4easyreg.com
Process Validation for Medical Devices Overview of FDA Requirements Label Validation Definition This paper begins a discussion on the varied ways to implement packaging validation. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation. Label Validation Definition.
From www.v7labs.com
Train Test Validation Split How To & Best Practices [2023] Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. This paper begins. Label Validation Definition.
From www.scribd.com
Validation of Pharmaceutical Packaging Verification And Validation Label Validation Definition Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. This paper. Label Validation Definition.
From www.qclabels.com
Validation Labels, Calibration Labels Label Validation Definition Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. This guidance document describes the general labelling principles. Label Validation Definition.
From www.slideserve.com
PPT TES Validation Results from the First Twelve Months and Planning Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. Focusing on the primary, secondary and tertiary packaging of drug products, it. Label Validation Definition.
From www.slideshare.net
Revised Process Validation Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. The basic principle of quality assurance. This paper begins a discussion on the varied ways to implement packaging validation. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. (z) validation means confirmation by examination and provision of. Label Validation Definition.
From www.slideserve.com
PPT Validating Requirements PowerPoint Presentation, free download Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. The basic principle of quality assurance. This paper begins a discussion on. Label Validation Definition.
From www.youtube.com
Difference between Verification and Validation ISO 9001 Definitions Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. This paper begins a discussion on the varied ways to implement packaging validation. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements. Label Validation Definition.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. This paper begins a discussion on the varied ways to implement. Label Validation Definition.
From www.youtube.com
How to Write a Validation Protocol Different Parts of Validation Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. Effective process validation contributes significantly to assuring drug quality. This paper begins a discussion on the varied ways to implement packaging validation. This guidance document describes the general. Label Validation Definition.
From read.cholonautas.edu.pe
What Is Form Validation And Its Types Printable Templates Free Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. Structured product. Label Validation Definition.
From www.dreamstime.com
Validation. a Text Label in the Business Planning Folder Stock Image Label Validation Definition This paper begins a discussion on the varied ways to implement packaging validation. The basic principle of quality assurance. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures. Structured product labeling (spl) implementation guide with validation procedures. This guidance document describes the general labelling principles for medical devices and ivd medical. Label Validation Definition.
From www.slideserve.com
PPT Process Validation General Principles and Practices PowerPoint Label Validation Definition Effective process validation contributes significantly to assuring drug quality. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. The basic principle of quality assurance. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. This paper begins a discussion on the varied ways. Label Validation Definition.
From www.vectorstock.com
Validation labels Royalty Free Vector Image VectorStock Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. Effective process validation contributes significantly to assuring drug quality. The basic principle of quality assurance. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and. Label Validation Definition.
From 360rto.com.au
What is Assessment Validation? How to Validate Assessment Tools 360RTO Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Effective process validation contributes significantly to assuring drug quality. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective. Label Validation Definition.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Label Validation Definition (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. Effective process. Label Validation Definition.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. This paper begins a discussion on the varied ways to implement packaging validation. The basic principle of quality assurance. This. Label Validation Definition.
From docs.aws.amazon.com
Automate Data Labeling Amazon SageMaker Label Validation Definition The basic principle of quality assurance. This paper begins a discussion on the varied ways to implement packaging validation. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product. Label Validation Definition.
From www.raqam.com
Product Label Validation Services (F&B, Cosmetics) RAQAM Consultancy Label Validation Definition Effective process validation contributes significantly to assuring drug quality. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. The. Label Validation Definition.
From www.slideserve.com
PPT Validation Part 5 Review and summary PowerPoint Presentation Label Validation Definition The basic principle of quality assurance. This paper begins a discussion on the varied ways to implement packaging validation. Structured product labeling (spl) implementation guide with validation procedures. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. (z) validation means confirmation by examination and provision of objective. Label Validation Definition.
From www.slideserve.com
PPT Validation and Validation Checks PowerPoint Presentation, free Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Effective process validation contributes significantly to assuring drug quality. This paper begins a discussion on the varied ways to implement packaging validation. Structured product. Label Validation Definition.
From www.unitedadlabel.com
Validation Label, 11/2" x 3/4" United Ad Label Label Validation Definition Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. This paper begins a discussion on. Label Validation Definition.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. This paper begins a discussion on the varied ways to implement packaging validation. Effective process validation contributes significantly to. Label Validation Definition.
From mudassiriqbal.net
What is Data Validation and Why It Is Necessary Mudassir Iqbal Label Validation Definition The basic principle of quality assurance. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Effective process validation contributes significantly to assuring drug quality. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Structured product labeling (spl). Label Validation Definition.
From www.vectorstock.com
Validation labels Royalty Free Vector Image VectorStock Label Validation Definition The basic principle of quality assurance. Structured product labeling (spl) implementation guide with validation procedures. Effective process validation contributes significantly to assuring drug quality. Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective evidence that the particular. Label Validation Definition.
From www.slideserve.com
PPT Validation of Pharmaceutical Packaging PowerPoint Presentation Label Validation Definition This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Structured product labeling (spl) implementation guide with validation procedures. The basic principle of quality assurance. Effective process validation contributes significantly to assuring drug quality. Structured product labeling (spl) implementation guide with validation procedures. Focusing on the primary, secondary and tertiary packaging. Label Validation Definition.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Label Validation Definition Structured product labeling (spl) implementation guide with validation procedures. Structured product labeling (spl) implementation guide with validation procedures. (z) validation means confirmation by examination and provision of objective evidence that the particular requirements for a. This paper begins a discussion on the varied ways to implement packaging validation. The basic principle of quality assurance. Effective process validation contributes significantly to. Label Validation Definition.
From mac51.blogspot.com
What are 4 must know Types of Validations in pharmaceutical industry FDA Label Validation Definition Focusing on the primary, secondary and tertiary packaging of drug products, it offers. Structured product labeling (spl) implementation guide with validation procedures. This paper begins a discussion on the varied ways to implement packaging validation. The basic principle of quality assurance. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier.. Label Validation Definition.