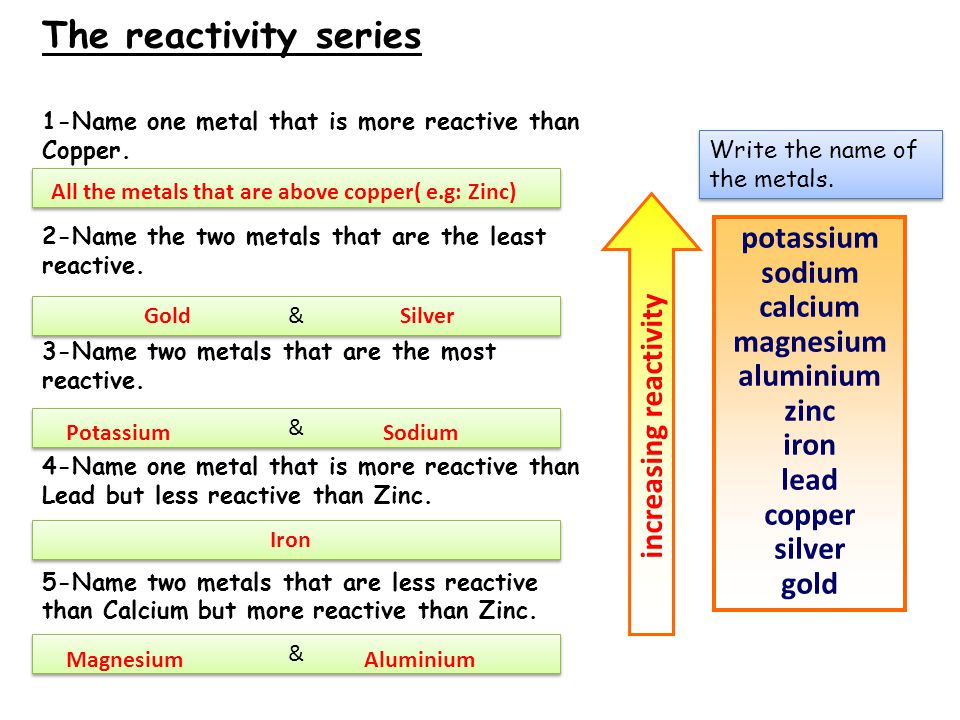
Magnesium Or Calcium More Reactive . Magnesium + hydrochloric acid →. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. Students will be able to discover that group 1 metals are more reactive. reaction between metals and water. for example, magnesium reacts rapidly with dilute hydrochloric acid: And steam enable us to put them into this series. More reactive metals displace less reactive. The tables show how the elements react with water and dilute acids: 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. 9 rows the reactivity series ranks metals by how readily they react. Potassium, sodium, and calcium all undergo reactions with. compare group 1 and group 2 metals with this practical that shows their reactivity rates.
from raphael-has-chung.blogspot.com
9 rows the reactivity series ranks metals by how readily they react. compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium + hydrochloric acid →. Students will be able to discover that group 1 metals are more reactive. for example, magnesium reacts rapidly with dilute hydrochloric acid: the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. And steam enable us to put them into this series. Potassium, sodium, and calcium all undergo reactions with. reaction between metals and water. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form.
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung
Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: compare group 1 and group 2 metals with this practical that shows their reactivity rates. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. Potassium, sodium, and calcium all undergo reactions with. Students will be able to discover that group 1 metals are more reactive. 9 rows the reactivity series ranks metals by how readily they react. Magnesium + hydrochloric acid →. And steam enable us to put them into this series. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. The tables show how the elements react with water and dilute acids: reaction between metals and water. More reactive metals displace less reactive. for example, magnesium reacts rapidly with dilute hydrochloric acid:
From material-properties.org
Magnesium and Calcium Comparison Properties Material Properties Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: Students will be able to discover that group 1 metals are more reactive. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical,. Magnesium Or Calcium More Reactive.
From www.pinterest.com
Twitter Health and nutrition, Natural health, Nutrition Magnesium Or Calcium More Reactive the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium + hydrochloric acid →. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. More reactive metals displace. Magnesium Or Calcium More Reactive.
From www.pinterest.com
NATURAL FACTORS Calcium Magnesium. Learn more about calcium magnesium Magnesium Or Calcium More Reactive compare group 1 and group 2 metals with this practical that shows their reactivity rates. Potassium, sodium, and calcium all undergo reactions with. And steam enable us to put them into this series. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. More reactive metals displace less. Magnesium Or Calcium More Reactive.
From www.differencebetween.com
Difference Between Calcium and Magnesium Compare the Difference Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: And steam enable us to put them into this series. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. 58. Magnesium Or Calcium More Reactive.
From www.walmart.com
NOW Supplements, Calcium & Magnesium with Vitamin D3 and Zinc Magnesium Or Calcium More Reactive Potassium, sodium, and calcium all undergo reactions with. The tables show how the elements react with water and dilute acids: Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. reaction between metals and water. And steam enable us to put them into this series. for example,. Magnesium Or Calcium More Reactive.
From www.tececo.com.au
TecEco Reactive Magnesia Magnesium Or Calcium More Reactive The tables show how the elements react with water and dilute acids: compare group 1 and group 2 metals with this practical that shows their reactivity rates. Students will be able to discover that group 1 metals are more reactive. for example, magnesium reacts rapidly with dilute hydrochloric acid: reaction between metals and water. And steam enable. Magnesium Or Calcium More Reactive.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Magnesium Or Calcium More Reactive compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium + hydrochloric acid →. More reactive metals displace less reactive. Students will be able to discover that group 1 metals are more reactive. Potassium, sodium, and calcium all undergo reactions with. the more reactive metals will react with cold water to form. Magnesium Or Calcium More Reactive.
From www.amazon.com
reactive magnesium Magnesium Or Calcium More Reactive More reactive metals displace less reactive. Potassium, sodium, and calcium all undergo reactions with. Magnesium + hydrochloric acid →. The tables show how the elements react with water and dilute acids: for example, magnesium reacts rapidly with dilute hydrochloric acid: 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. . Magnesium Or Calcium More Reactive.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Magnesium Or Calcium More Reactive 9 rows the reactivity series ranks metals by how readily they react. for example, magnesium reacts rapidly with dilute hydrochloric acid: Potassium, sodium, and calcium all undergo reactions with. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. Magnesium + hydrochloric acid →. And steam enable us to put. Magnesium Or Calcium More Reactive.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: More reactive metals displace less reactive. Potassium, sodium, and calcium all undergo reactions with. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. the more reactive metals will react with cold water to form metal hydroxide and. Magnesium Or Calcium More Reactive.
From www.jigsawhealth.com
Calcium and Magnesium The Dynamic Duo Magnesium Or Calcium More Reactive 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. The tables show how the elements react with water and dilute acids: for example, magnesium reacts rapidly with dilute hydrochloric acid: 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive. . Magnesium Or Calcium More Reactive.
From medium.com
What is Magnesium? Periodic Table Elements Medium Periodic Table Magnesium Or Calcium More Reactive Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. And steam enable us to put them into this series. Potassium, sodium, and calcium all undergo reactions with. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. More reactive metals displace. Magnesium Or Calcium More Reactive.
From www.dreamstime.com
Magnesium and Calcium in Balance Pictured As a Scale and Words Magnesium Or Calcium More Reactive The tables show how the elements react with water and dilute acids: reaction between metals and water. And steam enable us to put them into this series. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. 9 rows the reactivity series ranks metals by how readily they react. Calcium and. Magnesium Or Calcium More Reactive.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Magnesium Or Calcium More Reactive reaction between metals and water. 9 rows the reactivity series ranks metals by how readily they react. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. And steam enable us to put them into this series. for example, magnesium reacts rapidly with dilute hydrochloric acid:. Magnesium Or Calcium More Reactive.
From www.vrogue.co
Reactivity Series Of Metals O Level Chemistry Notes vrogue.co Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. More reactive metals displace less reactive. 9 rows the reactivity series ranks metals by how readily they react. Potassium, sodium, and calcium all undergo reactions with. And steam enable us to. Magnesium Or Calcium More Reactive.
From byjus.com
How Calcium or Magnesium can displace Sodium or Potassium from Magnesium Or Calcium More Reactive Magnesium + hydrochloric acid →. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. More reactive metals displace less reactive. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. compare group 1 and group 2 metals with this practical. Magnesium Or Calcium More Reactive.
From www.alamy.com
reactivity of different metals with hydrochloric acid calcium Stock Magnesium Or Calcium More Reactive reaction between metals and water. Magnesium + hydrochloric acid →. for example, magnesium reacts rapidly with dilute hydrochloric acid: the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. compare group 1 and group 2 metals with this practical that shows their reactivity rates. The tables show how the elements. Magnesium Or Calcium More Reactive.
From www.youtube.com
CHEMISTRY 9/CHAPTER 8/LECTURE 8/CHEMICAL REACTIVITY/ USES OF METALS Magnesium Or Calcium More Reactive Students will be able to discover that group 1 metals are more reactive. 9 rows the reactivity series ranks metals by how readily they react. for example, magnesium reacts rapidly with dilute hydrochloric acid: reaction between metals and water. compare group 1 and group 2 metals with this practical that shows their reactivity rates. Magnesium +. Magnesium Or Calcium More Reactive.
From www.adda247.com
Reactivity series of Metals and Non Metals Magnesium Or Calcium More Reactive the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. reaction between metals and water. The tables show how the elements react with water and dilute acids: compare group 1 and group 2 metals with this practical that shows their reactivity rates. More reactive metals displace less reactive. And steam enable. Magnesium Or Calcium More Reactive.
From blog.thepipingmart.com
Magnesium Properties and Uses Magnesium Or Calcium More Reactive Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. And steam enable us to put them into this series. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. Magnesium + hydrochloric acid →. compare group 1 and group. Magnesium Or Calcium More Reactive.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Magnesium Or Calcium More Reactive Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. 9 rows the reactivity series ranks metals by how readily they react. compare group 1 and group 2 metals with this practical that shows their reactivity rates. reaction between metals and water. More reactive metals displace. Magnesium Or Calcium More Reactive.
From dxobecmyv.blob.core.windows.net
Magnesium Atomic Number at Helen Day blog Magnesium Or Calcium More Reactive More reactive metals displace less reactive. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. reaction between metals and water. Students will be able to discover that group 1 metals are more reactive. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and.. Magnesium Or Calcium More Reactive.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Magnesium Or Calcium More Reactive Potassium, sodium, and calcium all undergo reactions with. And steam enable us to put them into this series. Students will be able to discover that group 1 metals are more reactive. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. reaction between metals and water. Magnesium + hydrochloric acid →. . Magnesium Or Calcium More Reactive.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Magnesium Or Calcium More Reactive More reactive metals displace less reactive. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. And steam enable us to put them into this series. Magnesium + hydrochloric acid →. 9 rows the reactivity series ranks metals by how readily they react. reaction between metals and. Magnesium Or Calcium More Reactive.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Magnesium Or Calcium More Reactive Magnesium + hydrochloric acid →. The tables show how the elements react with water and dilute acids: compare group 1 and group 2 metals with this practical that shows their reactivity rates. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. reaction between metals and water.. Magnesium Or Calcium More Reactive.
From raphael-has-chung.blogspot.com
Why Is Calcium More Reactive Than Magnesium RaphaelhasChung Magnesium Or Calcium More Reactive Magnesium + hydrochloric acid →. reaction between metals and water. 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. The tables show how the elements react with water and dilute acids: for example, magnesium reacts rapidly with dilute hydrochloric acid: compare group 1 and group 2 metals with. Magnesium Or Calcium More Reactive.
From www.youtube.com
Magnesium and Calcium (Part 3) Hypercalcemia Causes & How To Get Rid Magnesium Or Calcium More Reactive The tables show how the elements react with water and dilute acids: the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. Potassium, sodium, and calcium all undergo reactions with. And steam enable us to put them into this series. More reactive metals displace less reactive. reaction between metals and water. . Magnesium Or Calcium More Reactive.
From byjus.com
How Calcium or Magnesium can displace Sodium or Potassium from Magnesium Or Calcium More Reactive 9 rows the reactivity series ranks metals by how readily they react. Students will be able to discover that group 1 metals are more reactive. And steam enable us to put them into this series. Potassium, sodium, and calcium all undergo reactions with. the more reactive metals will react with cold water to form metal hydroxide and hydrogen. Magnesium Or Calcium More Reactive.
From estudyassistant.com
Which of the following elements are more reactive than the others. A Magnesium Or Calcium More Reactive Potassium, sodium, and calcium all undergo reactions with. compare group 1 and group 2 metals with this practical that shows their reactivity rates. The tables show how the elements react with water and dilute acids: Magnesium + hydrochloric acid →. for example, magnesium reacts rapidly with dilute hydrochloric acid: And steam enable us to put them into this. Magnesium Or Calcium More Reactive.
From thefitnessmanual.com
Is Magnesium More Reactive Than Calcium TheFitnessManual Magnesium Or Calcium More Reactive And steam enable us to put them into this series. 9 rows the reactivity series ranks metals by how readily they react. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. Magnesium + hydrochloric acid →. compare group 1 and group 2 metals with this practical. Magnesium Or Calcium More Reactive.
From www.walmart.com
Nature's Way Calcium and Magnesium Capsules, 100 Ct Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: Magnesium + hydrochloric acid →. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. Students will be able to discover that group 1 metals are more reactive. The tables show how the elements react with water and dilute acids: More reactive metals. Magnesium Or Calcium More Reactive.
From betapharmacy.ca
Jamieson Calcium Magnesium Beta Pharmacy Magnesium Or Calcium More Reactive for example, magnesium reacts rapidly with dilute hydrochloric acid: The tables show how the elements react with water and dilute acids: Students will be able to discover that group 1 metals are more reactive. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. 9 rows the reactivity series ranks metals. Magnesium Or Calcium More Reactive.
From quizizz.com
Reactivity Series Chemistry Quiz Quizizz Magnesium Or Calcium More Reactive Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form. The tables show how the elements react with water and dilute acids: Potassium, sodium, and calcium all undergo reactions with. Magnesium + hydrochloric acid →. 9 rows the reactivity series ranks metals by how readily they react. . Magnesium Or Calcium More Reactive.
From thepaleodiet.com
3 Micronutrient Ratios to Keep in Balance The Paleo Diet® Magnesium Or Calcium More Reactive The tables show how the elements react with water and dilute acids: for example, magnesium reacts rapidly with dilute hydrochloric acid: And steam enable us to put them into this series. Students will be able to discover that group 1 metals are more reactive. the more reactive metals will react with cold water to form metal hydroxide and. Magnesium Or Calcium More Reactive.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium Or Calcium More Reactive 58 rows in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and. the more reactive metals will react with cold water to form metal hydroxide and hydrogen gas. Magnesium + hydrochloric acid →. And steam enable us to put them into this series. Students will be able to discover that group 1 metals. Magnesium Or Calcium More Reactive.