Zinc Carbonate When Heated . zinc carbonate is a white powdery solid. Both carbonates and nitrates become more thermally stable as you go down the group. When it is heated strongly, it. The ones lower down have to be heated more strongly than those at. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. cid 23994 (zinc) dates. Zinc carbonate is the inorganic compound with the formula znco 3. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. It is a white solid that is insoluble in. Zinc carbonate appears as a white crystalline solid or powder that is. Decomposing is the process of breaking down. polarizing the carbonate ion. Thermal decomposition is a chemical.
from studylib.net
The ones lower down have to be heated more strongly than those at. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. cid 23994 (zinc) dates. polarizing the carbonate ion. zinc carbonate is a white powdery solid. Zinc carbonate is the inorganic compound with the formula znco 3. Decomposing is the process of breaking down. Thermal decomposition is a chemical. Zinc carbonate appears as a white crystalline solid or powder that is. It is a white solid that is insoluble in.
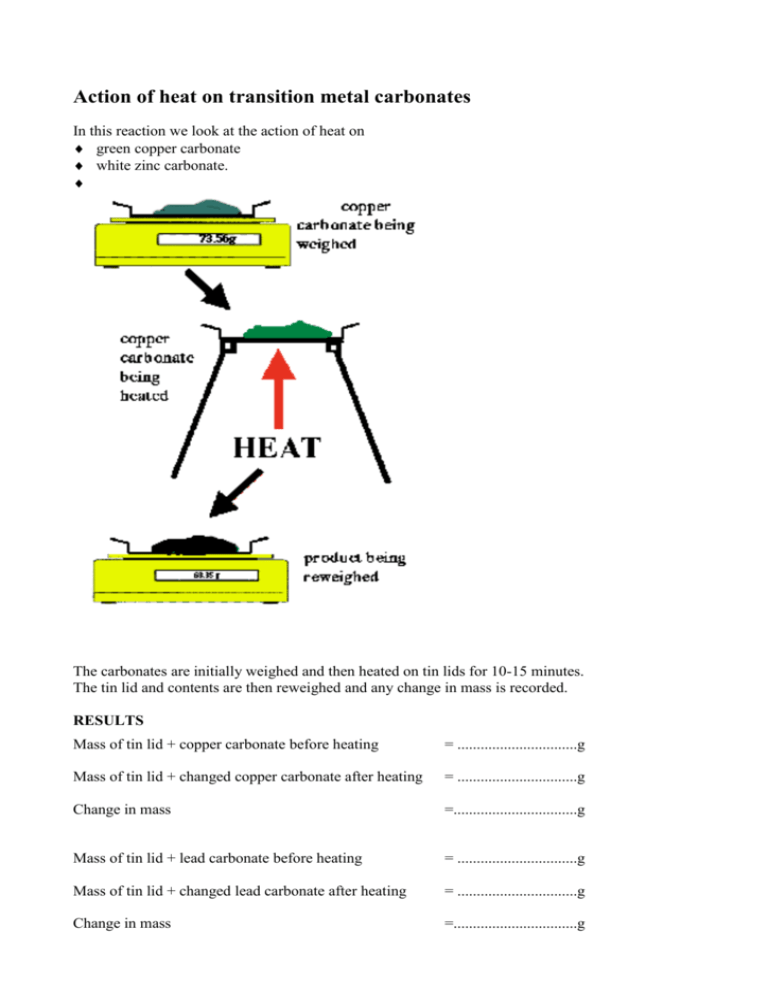
Action of heat on transition metal carbonates In
Zinc Carbonate When Heated When it is heated strongly, it. polarizing the carbonate ion. Both carbonates and nitrates become more thermally stable as you go down the group. cid 23994 (zinc) dates. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. When it is heated strongly, it. Decomposing is the process of breaking down. The ones lower down have to be heated more strongly than those at. zinc carbonate is a white powdery solid. Zinc carbonate is the inorganic compound with the formula znco 3. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Zinc carbonate appears as a white crystalline solid or powder that is. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. It is a white solid that is insoluble in. Thermal decomposition is a chemical.
From www.youtube.com
Heating zinc carbonate YouTube Zinc Carbonate When Heated Zinc carbonate appears as a white crystalline solid or powder that is. Decomposing is the process of breaking down. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Zinc carbonate is the inorganic compound with the formula znco 3. polarizing the carbonate ion. The ones lower down. Zinc Carbonate When Heated.
From testbook.com
Zinc Carbonate Learn its Formula, Structure, Properties & Uses Zinc Carbonate When Heated When it is heated strongly, it. Zinc carbonate is the inorganic compound with the formula znco 3. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of. Zinc Carbonate When Heated.
From www.youtube.com
QA Lab Practical Heating zinc carbonate YouTube Zinc Carbonate When Heated Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Decomposing is the process of breaking down. If this ion is placed next to a cation, such as. Zinc Carbonate When Heated.
From sielc.com
Zinc carbonate SIELC Zinc Carbonate When Heated Both carbonates and nitrates become more thermally stable as you go down the group. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Zinc carbonate appears as a white crystalline solid or powder that is. zinc carbonate is a white powdery solid. polarizing the carbonate ion.. Zinc Carbonate When Heated.
From studylib.net
Action of heat on transition metal carbonates In Zinc Carbonate When Heated Zinc carbonate is the inorganic compound with the formula znco 3. When it is heated strongly, it. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Both carbonates and nitrates become more thermally stable as you go down the group. polarizing the. Zinc Carbonate When Heated.
From www.coursehero.com
[Solved] Draw the heating curve for Zinc as it is heated from solid to Zinc Carbonate When Heated Zinc carbonate is the inorganic compound with the formula znco 3. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. It is a white solid that is insoluble in. Thermal decomposition is a chemical. zinc carbonate is a white powdery solid. . Zinc Carbonate When Heated.
From www.sciencephoto.com
Heating zinc in a flame Stock Image A510/0280 Science Photo Library Zinc Carbonate When Heated It is a white solid that is insoluble in. Zinc carbonate is the inorganic compound with the formula znco 3. The ones lower down have to be heated more strongly than those at. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. If this ion is placed next. Zinc Carbonate When Heated.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate When Heated Both carbonates and nitrates become more thermally stable as you go down the group. Zinc carbonate is the inorganic compound with the formula znco 3. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. The ones lower down have to be heated more. Zinc Carbonate When Heated.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The Zinc Carbonate When Heated cid 23994 (zinc) dates. Both carbonates and nitrates become more thermally stable as you go down the group. polarizing the carbonate ion. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the. Zinc Carbonate When Heated.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate When Heated some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. zinc carbonate is a white powdery solid. Decomposing is the process of breaking down. The ones lower down have to be heated more strongly than those at. Both carbonates and nitrates become more thermally stable as you go. Zinc Carbonate When Heated.
From slideplayer.com
Transition elements Zinc ppt download Zinc Carbonate When Heated Thermal decomposition is a chemical. Both carbonates and nitrates become more thermally stable as you go down the group. Decomposing is the process of breaking down. The ones lower down have to be heated more strongly than those at. It is a white solid that is insoluble in. polarizing the carbonate ion. Zinc carbonate is the inorganic compound with. Zinc Carbonate When Heated.
From brainly.in
Write chemical reaction for each (1) Zinc carbonate is calcinated. (2 Zinc Carbonate When Heated cid 23994 (zinc) dates. The ones lower down have to be heated more strongly than those at. It is a white solid that is insoluble in. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. If this ion is placed next to a cation, such as a. Zinc Carbonate When Heated.
From www.youtube.com
ACTION OF HEAT ON ZINC CARBONATE YouTube Zinc Carbonate When Heated When it is heated strongly, it. polarizing the carbonate ion. The ones lower down have to be heated more strongly than those at. Zinc carbonate is the inorganic compound with the formula znco 3. zinc carbonate is a white powdery solid. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of. Zinc Carbonate When Heated.
From www.slideserve.com
PPT SALT PowerPoint Presentation, free download ID4760975 Zinc Carbonate When Heated polarizing the carbonate ion. Thermal decomposition is a chemical. Zinc carbonate appears as a white crystalline solid or powder that is. Zinc carbonate is the inorganic compound with the formula znco 3. It is a white solid that is insoluble in. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate,. Zinc Carbonate When Heated.
From www.youtube.com
How to write chemical formula of zinc carbonateMolecular formula of Zinc Carbonate When Heated Thermal decomposition is a chemical. It is a white solid that is insoluble in. Zinc carbonate appears as a white crystalline solid or powder that is. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. Zinc carbonate is the inorganic compound with the formula znco 3. zinc. Zinc Carbonate When Heated.
From ceoxygdo.blob.core.windows.net
Zinc Carbonate at Melissa Henry blog Zinc Carbonate When Heated The ones lower down have to be heated more strongly than those at. polarizing the carbonate ion. cid 23994 (zinc) dates. Zinc carbonate is the inorganic compound with the formula znco 3. Zinc carbonate appears as a white crystalline solid or powder that is. Decomposing is the process of breaking down. It is a white solid that is. Zinc Carbonate When Heated.
From nanografi.com
Zinc Carbonate Properties and Applications Nanografi Nano Technology Zinc Carbonate When Heated zinc carbonate is a white powdery solid. Zinc carbonate appears as a white crystalline solid or powder that is. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. It is a white solid that is insoluble in. polarizing the carbonate ion.. Zinc Carbonate When Heated.
From ceoxygdo.blob.core.windows.net
Zinc Carbonate at Melissa Henry blog Zinc Carbonate When Heated polarizing the carbonate ion. zinc carbonate is a white powdery solid. The ones lower down have to be heated more strongly than those at. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Thermal decomposition is a chemical. When it is. Zinc Carbonate When Heated.
From www.semanticscholar.org
Figure 4 from Effect of heating on structure and leaching Zinc Carbonate When Heated It is a white solid that is insoluble in. The ones lower down have to be heated more strongly than those at. When it is heated strongly, it. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. polarizing the carbonate ion. Decomposing is the process of breaking. Zinc Carbonate When Heated.
From www.semanticscholar.org
Figure 1 from Effect of heating on structure and leaching Zinc Carbonate When Heated When it is heated strongly, it. cid 23994 (zinc) dates. Thermal decomposition is a chemical. Zinc carbonate appears as a white crystalline solid or powder that is. polarizing the carbonate ion. Decomposing is the process of breaking down. Both carbonates and nitrates become more thermally stable as you go down the group. It is a white solid that. Zinc Carbonate When Heated.
From www.youtube.com
How to Write the Formula for ZnCO3 (Zinc carbonate) YouTube Zinc Carbonate When Heated It is a white solid that is insoluble in. cid 23994 (zinc) dates. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. When it is heated strongly, it. Decomposing is the process of breaking down. The ones lower down have to be heated more strongly than those. Zinc Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound Zinc Carbonate When Heated Thermal decomposition is a chemical. Zinc carbonate appears as a white crystalline solid or powder that is. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. zinc carbonate is a white powdery solid. Compare the thermal stabilities of carbonates of reactive metals,. Zinc Carbonate When Heated.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Zinc Carbonate When Heated zinc carbonate is a white powdery solid. cid 23994 (zinc) dates. Thermal decomposition is a chemical. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. When it is heated strongly, it. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate,. Zinc Carbonate When Heated.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate When Heated If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. cid 23994 (zinc) dates. When it is heated strongly, it. Decomposing is the process of breaking down. Thermal decomposition is a chemical. Compare the thermal stabilities of carbonates of reactive metals, like sodium. Zinc Carbonate When Heated.
From www.youtube.com
Synthesis of basic Zinc Carbonate YouTube Zinc Carbonate When Heated zinc carbonate is a white powdery solid. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. Zinc carbonate is the inorganic. Zinc Carbonate When Heated.
From brainly.in
2.5g zinc carbonare is heated what is the mass of zinc oxide formed Zinc Carbonate When Heated When it is heated strongly, it. Both carbonates and nitrates become more thermally stable as you go down the group. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. It is a white solid that is insoluble in. Zinc carbonate appears as a. Zinc Carbonate When Heated.
From brainly.in
Write chemical reaction for each (1) Zinc carbonate is calcinated. (2 Zinc Carbonate When Heated It is a white solid that is insoluble in. Thermal decomposition is a chemical. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. Zinc carbonate appears as. Zinc Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound e book Zinc Carbonate When Heated It is a white solid that is insoluble in. Zinc carbonate is the inorganic compound with the formula znco 3. Decomposing is the process of breaking down. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Compare the thermal stabilities of carbonates of reactive metals, like sodium and. Zinc Carbonate When Heated.
From www.chemone.com
Zinc Carbonate Zinc Carbonate When Heated Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. Thermal decomposition is a chemical. The ones lower down have to be heated more strongly than those at. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons. Zinc Carbonate When Heated.
From www.youtube.com
Calculating Ksp of Zinc Carbonate using electrode potentials YouTube Zinc Carbonate When Heated Decomposing is the process of breaking down. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in the carbonate ion, drawing. Zinc carbonate is the inorganic compound with the formula znco 3. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead. Zinc Carbonate When Heated.
From www.numerade.com
12.5g of zinc carbonate is heated, it to make 8.1g of zinc Zinc Carbonate When Heated zinc carbonate is a white powdery solid. Zinc carbonate appears as a white crystalline solid or powder that is. Decomposing is the process of breaking down. polarizing the carbonate ion. Both carbonates and nitrates become more thermally stable as you go down the group. The ones lower down have to be heated more strongly than those at. When. Zinc Carbonate When Heated.
From www.slideshare.net
Action of heat on chemical compound Zinc Carbonate When Heated some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. zinc carbonate is a white powdery solid. Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including. It is a white solid that is insoluble in. Zinc. Zinc Carbonate When Heated.
From www.slideserve.com
PPT What is a chemical reaction? PowerPoint Presentation, free Zinc Carbonate When Heated some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Zinc carbonate is the inorganic compound with the formula znco 3. The ones lower down have to be heated more strongly than those at. zinc carbonate is a white powdery solid. Decomposing is the process of breaking down.. Zinc Carbonate When Heated.
From fphoto.photoshelter.com
science chemistry compound zinc oxide Fundamental Photographs The Zinc Carbonate When Heated When it is heated strongly, it. The ones lower down have to be heated more strongly than those at. It is a white solid that is insoluble in. Zinc carbonate is the inorganic compound with the formula znco 3. Decomposing is the process of breaking down. Zinc carbonate appears as a white crystalline solid or powder that is. Both carbonates. Zinc Carbonate When Heated.
From www.semanticscholar.org
Figure 2 from Effect of heating on structure and leaching Zinc Carbonate When Heated cid 23994 (zinc) dates. some examples of metal carbonates that undergo thermal decomposition reactions are copper (ii) carbonate, lead (ii) carbonate, and zinc carbonate. Zinc carbonate appears as a white crystalline solid or powder that is. If this ion is placed next to a cation, such as a group 2 ion, the cation attracts the delocalized electrons in. Zinc Carbonate When Heated.