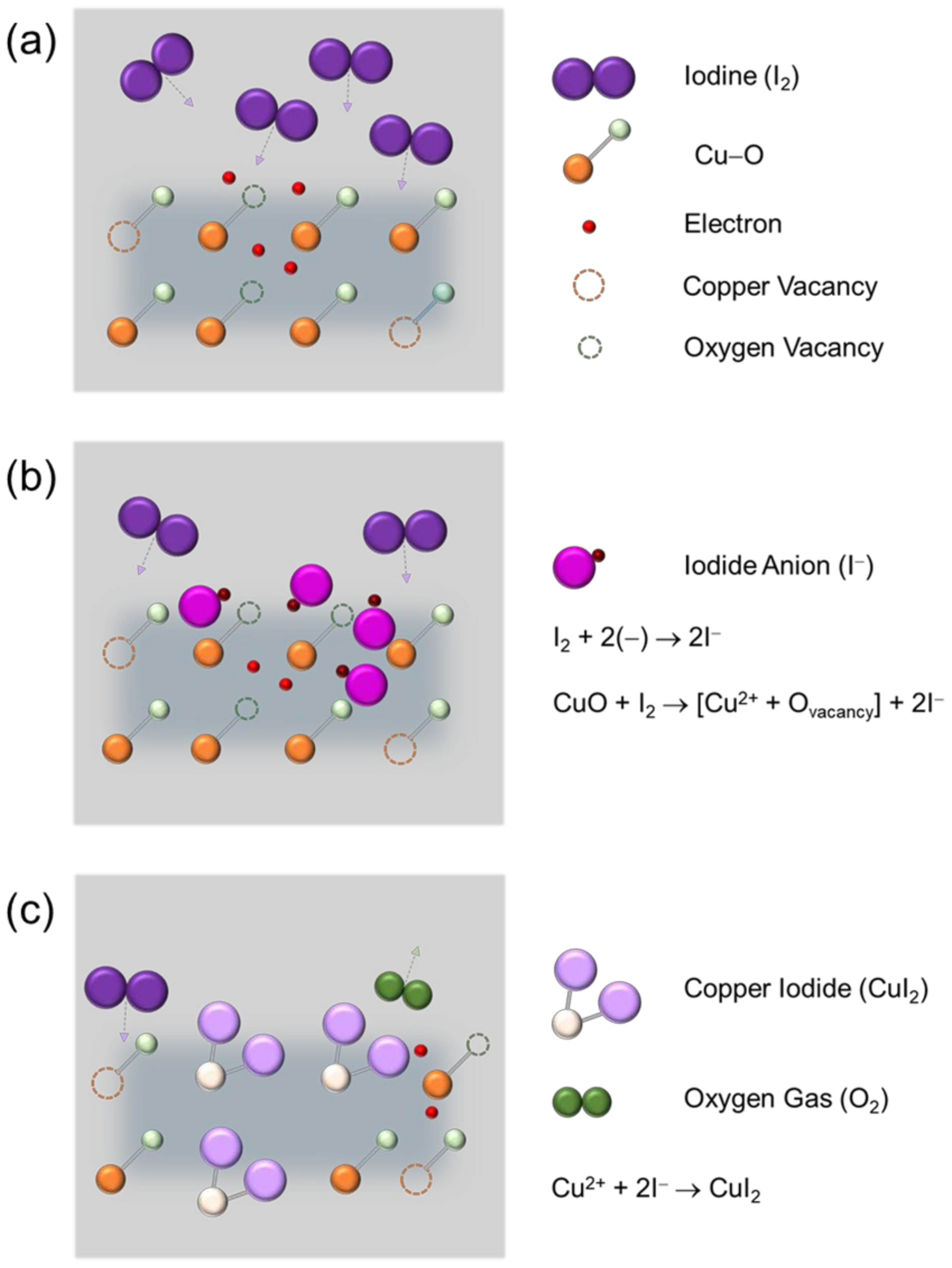
Copper Iodine Reaction . Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction of hexaaquacopper(ii) ions with iodide ions. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction can be represented by the following. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions.
from www.mdpi.com
The reaction of hexaaquacopper(ii) ions with iodide ions. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction can be represented by the following. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the.
Materials Free FullText Effects of Iodine Doping on Electrical
Copper Iodine Reaction The reaction can be represented by the following. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction can be represented by the following. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to.
From www.scribd.com
Copper I Iodide PDF Chemical Reactions Cysteine Copper Iodine Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The. Copper Iodine Reaction.
From pubs.acs.org
A Copper Iodide ClusterBased Coordination Polymer as an Unconventional Copper Iodine Reaction By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The. Copper Iodine Reaction.
From www.researchgate.net
Iodoniums and copper catalysis oxidative addition to Cu(I) and Copper Iodine Reaction A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction of hexaaquacopper(ii) ions with iodide ions. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell. Copper Iodine Reaction.
From www.youtube.com
Copper Chemistry Cu(I)Iodide (Redox) YouTube Copper Iodine Reaction The reaction can be represented by the following. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper(ii). Copper Iodine Reaction.
From www.researchgate.net
Copper iodide catalyzed synthesis of aryl cyanide using benzyl cyanide Copper Iodine Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction can be represented by the following. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. A general chemistry laboratory project involving the. Copper Iodine Reaction.
From www.youtube.com
Reaction of Copper sulfate (CuSO4) with Potassium iodide (KI) YouTube Copper Iodine Reaction Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction can be represented by the following. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction of hexaaquacopper(ii) ions with iodide ions.. Copper Iodine Reaction.
From www.slideserve.com
PPT Chem 3024 Fall 2003 Iodometric Determination of Copper PowerPoint Copper Iodine Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? The reaction can be represented by the following. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to.. Copper Iodine Reaction.
From www.youtube.com
Lewis Structure of CuI2, copper (II) iodide YouTube Copper Iodine Reaction The reaction can be represented by the following. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to. Copper Iodine Reaction.
From www.youtube.com
How to Write the Formula for Copper (I) iodide YouTube Copper Iodine Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper(ii) ions oxidise iodide ions to iodine,. Copper Iodine Reaction.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image A500/0573 Science Photo Copper Iodine Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction can be represented by the following. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to molecular iodine,. Copper Iodine Reaction.
From studylib.net
Copper Iodine Lab MAEDA AP Chemistry Copper Iodine Reaction A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Why does the reaction between copper(ii) ion and iodide. Copper Iodine Reaction.
From www.youtube.com
Copper Reacting with Iodine YouTube Copper Iodine Reaction Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. The reaction can be represented by the following. The reaction of hexaaquacopper(ii) ions with iodide ions. The. Copper Iodine Reaction.
From www.youtube.com
Iodine and Copper II Chloride YouTube Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction can be represented by the following. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The reaction of hexaaquacopper(ii) ions with iodide ions. Why does the reaction between copper(ii) ion. Copper Iodine Reaction.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image C030/7215 Science Photo Copper Iodine Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions.. Copper Iodine Reaction.
From www.youtube.com
How to Balance CuSO4 + KI = CuI + I2 + K2SO4 Copper (II) sulfate Copper Iodine Reaction Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction of hexaaquacopper(ii) ions with iodide ions.. Copper Iodine Reaction.
From www.chegg.com
Solved The primary route for making copper iodide is by Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Why does the reaction between copper(ii) ion and. Copper Iodine Reaction.
From www.researchgate.net
Copper iodide/DABCO/PivOH catalyzed process for Copper Iodine Reaction The reaction can be represented by the following. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The reaction of. Copper Iodine Reaction.
From www.toppr.com
What happen when?Copper sulphate reacts with potassium iodide. Copper Iodine Reaction The reaction can be represented by the following. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. By the redox reaction of copper(ii) cations with. Copper Iodine Reaction.
From www.youtube.com
CuSO4+KI=CuI+I2+K2SO4 Balanced EquationCopper sulphate+Potassium Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction can be represented by the following. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative?. Copper Iodine Reaction.
From www.chegg.com
Name of the Experiment Estimation of copper in the Copper Iodine Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction of hexaaquacopper(ii) ions with iodide ions. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates,. Copper Iodine Reaction.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodine Reaction Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Why does the reaction between copper(ii) ion and iodide ion. Copper Iodine Reaction.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodine Reaction Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. By. Copper Iodine Reaction.
From www.numerade.com
SOLVED Aqueous copper(II) ion reacts with aqueous iodide ion to yield Copper Iodine Reaction The reaction can be represented by the following. The reaction of hexaaquacopper(ii) ions with iodide ions. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper(ii) ions oxidize iodide ions to molecular iodine,. Copper Iodine Reaction.
From www.numerade.com
Aqueous copper(II) ion reacts with aqueous iodide ion to yield solid Copper Iodine Reaction The reaction can be represented by the following. The reaction of hexaaquacopper(ii) ions with iodide ions. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves. Copper Iodine Reaction.
From www.semanticscholar.org
Figure 1 from Selective copper(II) acetate and potassium iodide Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced. Copper Iodine Reaction.
From www.mdpi.com
Materials Free FullText Effects of Iodine Doping on Electrical Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. Copper iodide can be prepared in a laboratory by. Copper Iodine Reaction.
From www.researchgate.net
(PDF) Deposition of copper iodide thin films by chemical bath Copper Iodine Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. By the redox reaction of copper(ii). Copper Iodine Reaction.
From www.slideserve.com
PPT Chem 3024 Fall 2003 Iodometric Determination of Copper PowerPoint Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative?. Copper Iodine Reaction.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID3280712 Copper Iodine Reaction Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction can be represented by the following.. Copper Iodine Reaction.
From www.degruyter.com
Grignard reagents and Copper Copper Iodine Reaction The reaction can be represented by the following. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. By the redox reaction of copper(ii) cations with iodide anions the elemental iodine precipitates, which is responsible for colour variations of the. The reaction of hexaaquacopper(ii) ions with iodide ions. Why does the reaction between copper(ii) ion. Copper Iodine Reaction.
From www.researchgate.net
Development of a coupling between alkyl iodides and Nnucleophiles by Copper Iodine Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Why. Copper Iodine Reaction.
From www.youtube.com
Empirical Formula of Copper Iodide Virtual Lab YouTube Copper Iodine Reaction A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The reaction can be represented by the following. The reaction of. Copper Iodine Reaction.
From www.researchgate.net
Copper iodide/DABCO/PivOH catalyzed process for Copper Iodine Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction of hexaaquacopper(ii) ions with iodide ions. A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. The reaction can be represented by the following. Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative?. Copper Iodine Reaction.
From www.researchgate.net
Scheme 2 Synthesis of copper(I) iodide complex 1 (the molecular Copper Iodine Reaction A general chemistry laboratory project involving the synthesis and analysis of copper(i) iodide is described. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction of hexaaquacopper(ii) ions with iodide ions. Why does the reaction between copper(ii). Copper Iodine Reaction.
From www.youtube.com
Empirical Formula of Copper Iodide Virtual Lab Student B YouTube Copper Iodine Reaction Why does the reaction between copper(ii) ion and iodide ion proceed even though the e_cell of the set up is negative? Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are. The reaction can. Copper Iodine Reaction.