Medical Device Label Testing . Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how health canada inspects licensed medical device establishments (companies) in canada. Choose eurofins medical device testing to help you: This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Find out the meaning and examples of symbols for manufacturing,. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device.
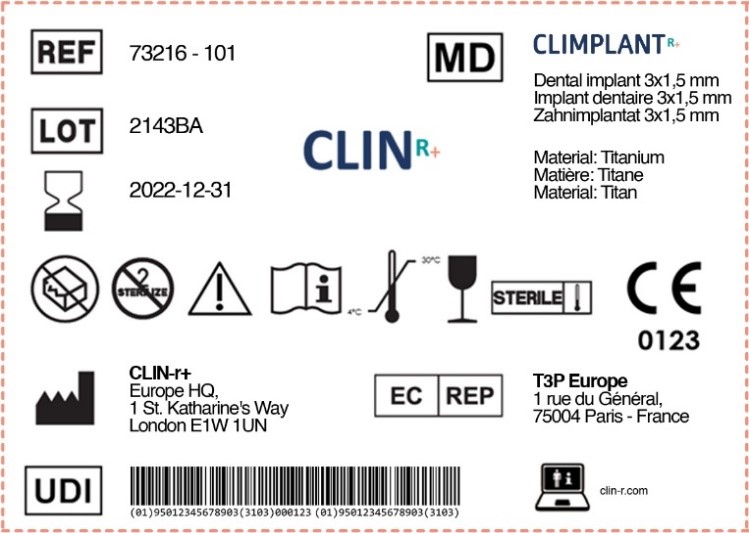
from clin-r.com
Find out the meaning and examples of symbols for manufacturing,. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Learn how health canada inspects licensed medical device establishments (companies) in canada. Choose eurofins medical device testing to help you: Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,.
Labels for Medical Devices Clin R
Medical Device Label Testing Find out the meaning and examples of symbols for manufacturing,. Choose eurofins medical device testing to help you: This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Find out the meaning and examples of symbols for manufacturing,. Learn how health canada inspects licensed medical device establishments (companies) in canada. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device.
From www.thelabelpeople.co.uk
PAT Test Labels Super Fast Delivery on all PAT Testing Labels Medical Device Label Testing Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Find out the meaning and examples of symbols for manufacturing,. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how. Medical Device Label Testing.
From sterilogix.com
Durability Testing of UDI Labeling SteriLogix Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. This document provides globally harmonized labelling principles for medical devices, including in. Medical Device Label Testing.
From satoasiapacific.com
SATO Medical Device Barcode Labelling Solution SATO AutoID Malaysia Medical Device Label Testing Choose eurofins medical device testing to help you: Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Find out the meaning and examples of. Medical Device Label Testing.
From printabletemplate.conaresvirtual.edu.sv
Medical Device Label Template Medical Device Label Testing Choose eurofins medical device testing to help you: Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique. Medical Device Label Testing.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Medical Device Label Testing Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu. Medical Device Label Testing.
From sabremedical.com.au
TESTING & STERILISATION Sabre Medical Medical Device Packaging Experts Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Find out the meaning and examples of symbols for manufacturing,.. Medical Device Label Testing.
From www.ccit.com
Why is Seal Integrity Testing of Medical Device Packaging Important Medical Device Label Testing Choose eurofins medical device testing to help you: Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Find out the meaning and examples of symbols for manufacturing,. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. This post will discuss what counts as a. Medical Device Label Testing.
From www.dreamstime.com
Collection Tube with Test Label on Medical Chart with Personal Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Choose eurofins medical device testing to help you: Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn how medical device manufacturers. Medical Device Label Testing.
From www.dreamstime.com
Collection Tube with Test Label on Medical Chart with Personal P Stock Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Find out the meaning and examples of symbols for manufacturing,. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Choose eurofins medical device. Medical Device Label Testing.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Label Testing Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Find out the meaning and examples of symbols for manufacturing,. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Choose eurofins. Medical Device Label Testing.
From www.regdesk.co
FDA Guidance on Testing and Labeling Medical Devices for Safety in the Medical Device Label Testing Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Learn how health canada inspects licensed medical device establishments (companies) in canada. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations.. Medical Device Label Testing.
From www.resourcelabel.com
Traceable Medical Device Labeling Resource Label Group Medical Device Label Testing This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. This post will discuss. Medical Device Label Testing.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Label Testing Choose eurofins medical device testing to help you: Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Find out the meaning and examples of symbols for manufacturing,. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs. Medical Device Label Testing.
From sterilogix.com
Durability Testing of UDI Labeling SteriLogix Medical Device Label Testing Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Learn how health canada inspects licensed medical device establishments (companies) in canada. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This document provides globally harmonized labelling principles for medical devices, including in vitro. Medical Device Label Testing.
From www.labtag.com
Clinical Trial Labels LabTAG Laboratory Labels Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Find out the meaning and examples of symbols for manufacturing,. This post will discuss what counts as a medical device label, where they are required, and look at the key. Medical Device Label Testing.
From stock.adobe.com
Full set of medical device packaging symbols with warning information Medical Device Label Testing Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Choose eurofins medical device testing to help you: This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. This post will discuss what counts as a medical device label, where they are required, and look at. Medical Device Label Testing.
From www.scilife.io
Relationship between usability testing and labeling of your medical Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Choose eurofins medical device testing to help you: Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and. Medical Device Label Testing.
From www.scilife.io
Relationship between usability testing and labeling of your medical Medical Device Label Testing Find out the meaning and examples of symbols for manufacturing,. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Learn how health canada inspects licensed medical device establishments (companies) in canada. This post will discuss what counts as a medical device label, where they are required, and look at the key points of. Medical Device Label Testing.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Find out the meaning and examples of symbols for manufacturing,. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Learn how medical device. Medical Device Label Testing.
From www.amazon.com
AAMI TIR122010, Designining, testing and labeling reusable medical Medical Device Label Testing Find out the meaning and examples of symbols for manufacturing,. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how health canada inspects licensed medical device establishments (companies) in canada. Choose eurofins medical device testing to help you: Learn. Medical Device Label Testing.
From knobbemedical.com
Capture2 Knobbe Medical Medical Device Label Testing Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This post will discuss what counts. Medical Device Label Testing.
From www.youtube.com
Medical testing kit QR code printing and labeling YouTube Medical Device Label Testing Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Find out the meaning and examples of symbols for manufacturing,. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. This post will discuss what counts as a medical device label, where they. Medical Device Label Testing.
From compliancetesting.com
FCC Labeling Requirements Compliance Testing Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Assess the robustness. Medical Device Label Testing.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Choose eurofins medical device testing to help you: Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Assess the robustness of. Medical Device Label Testing.
From array.aami.org
Designing, testing, and labeling medical devices intended for Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Find out the meaning and examples of symbols for manufacturing,. Learn how. Medical Device Label Testing.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Medical Device Label Testing Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Assess the robustness of your labels to. Medical Device Label Testing.
From westpak.com
Pharmaceutical Label Testing Westpak Medical Device Label Testing Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Find out the meaning and examples of symbols for manufacturing,. Assess the robustness. Medical Device Label Testing.
From blog.airlinehyd.com
Unlocking Expertise Free Online Training for Medical Device Labeling Medical Device Label Testing Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Find out the meaning and examples of symbols for manufacturing,. This post will discuss what counts as a medical device label, where they are required,. Medical Device Label Testing.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. This document provides globally harmonized labelling principles for medical devices, including in. Medical Device Label Testing.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Label Testing Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical. Medical Device Label Testing.
From www.microscan.com
FDA UDI Rule for Medical Devices Medical Device Label Testing This document provides globally harmonized labelling principles for medical devices, including in vitro diagnostic (ivd) medical devices, and. Find out the meaning and examples of symbols for manufacturing,. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn about the fda's labeling requirements and guidance for medical devices, including advertising,. Medical Device Label Testing.
From www.regdesk.co
FDA Guidance on Testing and Labeling Medical Devices for Safety in the Medical Device Label Testing Find out the meaning and examples of symbols for manufacturing,. Learn how health canada inspects licensed medical device establishments (companies) in canada. Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. This document provides. Medical Device Label Testing.
From www.hpnonline.com
FDA provides guidance on testing, labeling medical devices for safety Medical Device Label Testing Learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. Choose eurofins medical device testing to help you: This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Assess the robustness of your labels to. Medical Device Label Testing.
From cnrecare.en.made-in-china.com
Medical Professional Supply OEM Private Label Ovulation Testing Device Medical Device Label Testing Assess the robustness of your labels to verify that your barcodes will remain readable after being subjected to the. Learn how health canada inspects licensed medical device establishments (companies) in canada. Choose eurofins medical device testing to help you: Learn how medical device manufacturers must meet the gmp labeling requirements of the qs regulation, such as legibility, adhesion,. Learn about. Medical Device Label Testing.
From www.rigelmedical.com
Definitions & symbols used in IEC 60601 & 62353 Rigel Medical Medical Device Label Testing This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Find out the meaning and examples of symbols for manufacturing,. Choose eurofins medical device testing to help you: This document provides globally harmonized labelling principles for medical devices, including in vitro. Medical Device Label Testing.