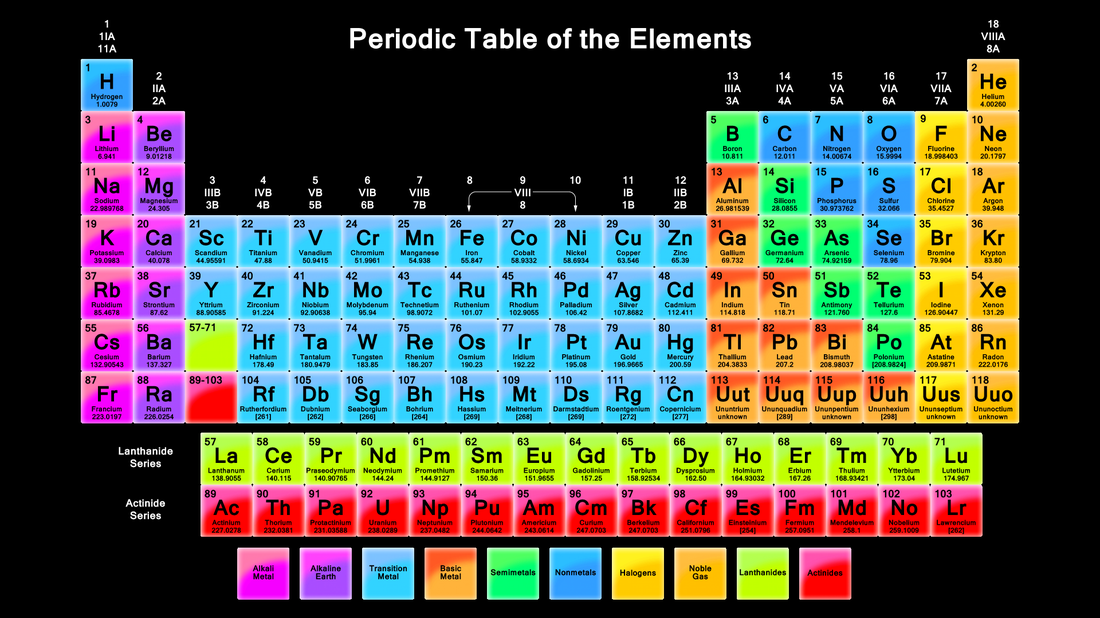
Metals Science Definition Periodic Table . The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Interactive periodic table showing names, electrons, and oxidation states. Metals are defined as those elements which have characteristic chemical and physical properties. Visualize trends, 3d orbitals, isotopes, and mix compounds. Elements that are not metals. The familiar types of metals are (iron,. Metals are on the left side of the periodic table. Nonmetals are on the right side of the table. This list contains the properties of metals, metalloids and nonmetals. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). The periodic table shows which elements are considered part of each group.
from wootensciencedesk.weebly.com
The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Interactive periodic table showing names, electrons, and oxidation states. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). This list contains the properties of metals, metalloids and nonmetals. The familiar types of metals are (iron,. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Nonmetals are on the right side of the table. Metals are on the left side of the periodic table. Visualize trends, 3d orbitals, isotopes, and mix compounds. Elements that are not metals.
Introduction to the Periodic Table of Elements Wooten's Science Desk
Metals Science Definition Periodic Table Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Nonmetals are on the right side of the table. Visualize trends, 3d orbitals, isotopes, and mix compounds. Metals are defined as those elements which have characteristic chemical and physical properties. The familiar types of metals are (iron,. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. The periodic table shows which elements are considered part of each group. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). This list contains the properties of metals, metalloids and nonmetals. Interactive periodic table showing names, electrons, and oxidation states. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are on the left side of the periodic table. Elements that are not metals.
From www.pinterest.com
Periodic Table Of Elements With Names And Symbols Chemistry Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Interactive periodic table showing names, electrons, and oxidation states. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Metals are on the left side of the periodic table. The familiar types of metals are (iron,. This list. Metals Science Definition Periodic Table.
From www.wpclipart.com
periodic table of the elements /science/atoms_molecules/periodic Metals Science Definition Periodic Table A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). This list contains the properties of metals, metalloids and nonmetals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are defined as those elements which have characteristic chemical and physical properties. The familiar types of metals. Metals Science Definition Periodic Table.
From theconversation.com
Understanding the periodic table through the lens of the volatile Group Metals Science Definition Periodic Table This list contains the properties of metals, metalloids and nonmetals. The familiar types of metals are (iron,. Visualize trends, 3d orbitals, isotopes, and mix compounds. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Metals are defined as those elements which have characteristic chemical and physical properties. Metals are on the. Metals Science Definition Periodic Table.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Metals Science Definition Periodic Table Elements that are not metals. Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3d orbitals, isotopes, and mix compounds. The periodic table shows which elements are considered part of each group. The familiar types of metals are (iron,. Metals are defined as those elements which have characteristic chemical and physical properties. This list contains the properties of. Metals Science Definition Periodic Table.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica Metals Science Definition Periodic Table A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Elements that are not metals. Visualize trends, 3d orbitals, isotopes, and mix compounds. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Interactive periodic table showing names, electrons, and oxidation states. This list contains the properties of. Metals Science Definition Periodic Table.
From sciencenotes.org
Printable Periodic Tables Science Notes and Projects Metals Science Definition Periodic Table Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Visualize trends, 3d orbitals, isotopes, and mix compounds. The periodic table shows which elements are considered part of each group. Metals are defined as those elements which have characteristic chemical and physical properties. Metals are. Metals Science Definition Periodic Table.
From sciencenotes.org
How to Use a Periodic Table Metals Science Definition Periodic Table Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Nonmetals are on the right side of the table. Elements that are not metals. A substance with high electrical conductivity, luster,. Metals Science Definition Periodic Table.
From safetyzik.weebly.com
Metals periodic table safetyzik Metals Science Definition Periodic Table Metals are on the left side of the periodic table. Nonmetals are on the right side of the table. Interactive periodic table showing names, electrons, and oxidation states. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Visualize trends, 3d orbitals, isotopes, and mix. Metals Science Definition Periodic Table.
From homedeso.vercel.app
What Is The Periodic Table Of Elements Definition Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. This list contains the properties of metals, metalloids and nonmetals. Interactive periodic table showing names, electrons, and oxidation states. Elements that are not metals. The periodic table shows which elements are considered part of each group. A substance with high electrical conductivity, luster, and malleability, which. Metals Science Definition Periodic Table.
From utedzz.blogspot.com
Periodic Table Of Elements Families And Groups Periodic Table Timeline Metals Science Definition Periodic Table A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). This list contains the properties of metals, metalloids and nonmetals. The familiar types of metals are (iron,. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light.. Metals Science Definition Periodic Table.
From sciencenotes.org
Representative Elements on the Periodic Table Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. This list contains the properties of metals, metalloids and nonmetals. The periodic table shows which elements are considered part of each group. Nonmetals are on the right side of the table. Metals are on the left side of the periodic table. A substance with high electrical. Metals Science Definition Periodic Table.
From www.animalia-life.club
Periodic Table Of Elements Transition Metals Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. The familiar types of metals are (iron,. Metals are defined as those elements which have characteristic chemical and physical properties. Visualize trends, 3d orbitals, isotopes, and mix compounds. Metals are on the left side of the periodic table. Metal, any of a class of substances characterized. Metals Science Definition Periodic Table.
From www.chemeurope.com
The Complete Periodic Table of Elements Metals Science Definition Periodic Table Elements that are not metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. This list contains the properties of metals, metalloids and nonmetals. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). The periodic. Metals Science Definition Periodic Table.
From tableformeonly.blogspot.com
What Are The Transition Metals On The Periodic Table Metals Science Definition Periodic Table A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Metals are on the left side of the periodic table. Interactive periodic table showing names, electrons, and oxidation states. Nonmetals are on the right side of the table. Metal, any of a class of substances characterized by high electrical and thermal conductivity. Metals Science Definition Periodic Table.
From www.ebay.com
Innovating Science 8x4' Giant Vinyl Periodic Table of the Elements Metals Science Definition Periodic Table Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Nonmetals are on the right side of the table. This list contains the properties of metals, metalloids and nonmetals. Metals are on the left side of the periodic table. Elements that are not metals. A. Metals Science Definition Periodic Table.
From www.lazada.com.ph
ALL ABOUT SCIENCE Laminated Chart for Kids PERIODIC TABLE OF ELEMENTS Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Elements that are not metals. Interactive periodic table showing names, electrons, and oxidation states. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. This list contains the properties of metals,. Metals Science Definition Periodic Table.
From www.britannica.com
Chemical compound Definition, Examples, & Types Britannica Metals Science Definition Periodic Table Metals are defined as those elements which have characteristic chemical and physical properties. Interactive periodic table showing names, electrons, and oxidation states. Elements that are not metals. This list contains the properties of metals, metalloids and nonmetals. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). The familiar types of metals. Metals Science Definition Periodic Table.
From esplora.org.mt
Oh Chemistry! Oh Chemistry! « Esplora Metals Science Definition Periodic Table Nonmetals are on the right side of the table. The familiar types of metals are (iron,. Interactive periodic table showing names, electrons, and oxidation states. Metals are defined as those elements which have characteristic chemical and physical properties. Metals are on the left side of the periodic table. Elements that are not metals. This list contains the properties of metals,. Metals Science Definition Periodic Table.
From www.pinterest.es
Color and Learn About the Periodic Table Layers of Learning Metals Science Definition Periodic Table Elements that are not metals. Interactive periodic table showing names, electrons, and oxidation states. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. The periodic table shows which elements are considered part of each group. Nonmetals are on the right side of the table.. Metals Science Definition Periodic Table.
From glossary.periodni.com
Periodic table Chemistry Dictionary & Glossary Metals Science Definition Periodic Table Metals are defined as those elements which have characteristic chemical and physical properties. This list contains the properties of metals, metalloids and nonmetals. Visualize trends, 3d orbitals, isotopes, and mix compounds. Nonmetals are on the right side of the table. Interactive periodic table showing names, electrons, and oxidation states. A substance with high electrical conductivity, luster, and malleability, which readily. Metals Science Definition Periodic Table.
From sciencewithmsbarton.wordpress.com
Elements Science with Mrs. Barton Metals Science Definition Periodic Table The familiar types of metals are (iron,. Interactive periodic table showing names, electrons, and oxidation states. Elements that are not metals. Nonmetals are on the right side of the table. This list contains the properties of metals, metalloids and nonmetals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility,. Metals Science Definition Periodic Table.
From philschatz.com
The Periodic Table of Elements · Biology Metals Science Definition Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Metals are defined as those elements which have characteristic chemical and physical properties. This list contains the properties of metals, metalloids and nonmetals. The familiar types of metals are (iron,. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Metals are on the. Metals Science Definition Periodic Table.
From infinitylearn.com
Modern Periodic Table, Law, Group, Elements and Atomic Number Metals Science Definition Periodic Table Interactive periodic table showing names, electrons, and oxidation states. The familiar types of metals are (iron,. Nonmetals are on the right side of the table. Metals are defined as those elements which have characteristic chemical and physical properties. Elements that are not metals. The periodic table shows which elements are considered part of each group. Metals are on the left. Metals Science Definition Periodic Table.
From education-portal.com
Representative Elements of the Periodic Table Definition & Overview Metals Science Definition Periodic Table Interactive periodic table showing names, electrons, and oxidation states. Elements that are not metals. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Metals are defined as those elements which have characteristic chemical and physical properties. The periodic table shows which elements are considered part of each group. The metals consist. Metals Science Definition Periodic Table.
From sciencenotes.org
What Are Noble Metals? Definition and List Metals Science Definition Periodic Table Nonmetals are on the right side of the table. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Interactive periodic table showing names, electrons, and oxidation states. The familiar types of metals are (iron,. Metals are on the left side of the periodic table. Metals are defined as those elements which. Metals Science Definition Periodic Table.
From sciencenotes.org
Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects Metals Science Definition Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Nonmetals are on the right side of the table. Metals are on the. Metals Science Definition Periodic Table.
From youngandrefined.com
Periodic Table Chart of the Elements Chart Laminated Classroom Poster Metals Science Definition Periodic Table The periodic table shows which elements are considered part of each group. This list contains the properties of metals, metalloids and nonmetals. Interactive periodic table showing names, electrons, and oxidation states. Metals are on the left side of the periodic table. Visualize trends, 3d orbitals, isotopes, and mix compounds. The metals consist of the alkali metals, alkaline earths, transition metals,. Metals Science Definition Periodic Table.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 ac Metals and nonmetals Metals Science Definition Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. This list contains the properties of metals, metalloids and nonmetals. Elements that are. Metals Science Definition Periodic Table.
From sciencenotes.org
Main Group Elements Definition and Importance Metals Science Definition Periodic Table Nonmetals are on the right side of the table. Visualize trends, 3d orbitals, isotopes, and mix compounds. This list contains the properties of metals, metalloids and nonmetals. Metals are on the left side of the periodic table. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are defined as those elements which have characteristic. Metals Science Definition Periodic Table.
From www.aiophotoz.com
Printable Periodic Table Of Elements With Names Science Struck Images Metals Science Definition Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Nonmetals are on the right side of the table. The familiar types of metals are (iron,. Elements that are not metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. A substance with high electrical conductivity,. Metals Science Definition Periodic Table.
From wootensciencedesk.weebly.com
Introduction to the Periodic Table of Elements Wooten's Science Desk Metals Science Definition Periodic Table A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Elements that are not metals. The periodic table shows which elements are considered part of each group. Metals are on the left side of the periodic table. Metals. Metals Science Definition Periodic Table.
From tagdiki.weebly.com
Metals on the periodic table tagdiki Metals Science Definition Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations). Nonmetals are on the right side of the table. Interactive periodic table showing names, electrons, and oxidation states. The familiar. Metals Science Definition Periodic Table.
From leecountytoday.blogspot.com
Periodic Table Of Elements Metals And Non Metals Decoration For Wedding Metals Science Definition Periodic Table Metals are defined as those elements which have characteristic chemical and physical properties. Elements that are not metals. The familiar types of metals are (iron,. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Nonmetals are on the right side of the table. Metal, any of a class of substances characterized by high electrical and. Metals Science Definition Periodic Table.
From sciencenotes.org
Printable Periodic Table Chart 2015 Metals Science Definition Periodic Table Elements that are not metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. The familiar types of metals are (iron,. This list contains the properties of metals, metalloids and nonmetals. Nonmetals are on the right side of the table. Visualize trends, 3d orbitals, isotopes, and mix compounds. Metals are on the left side of. Metals Science Definition Periodic Table.
From www.ducksters.com
Kids science Periodic Table of Elements Metals Science Definition Periodic Table The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are defined as those elements which have characteristic chemical and physical properties. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. Interactive periodic table showing names, electrons, and oxidation. Metals Science Definition Periodic Table.