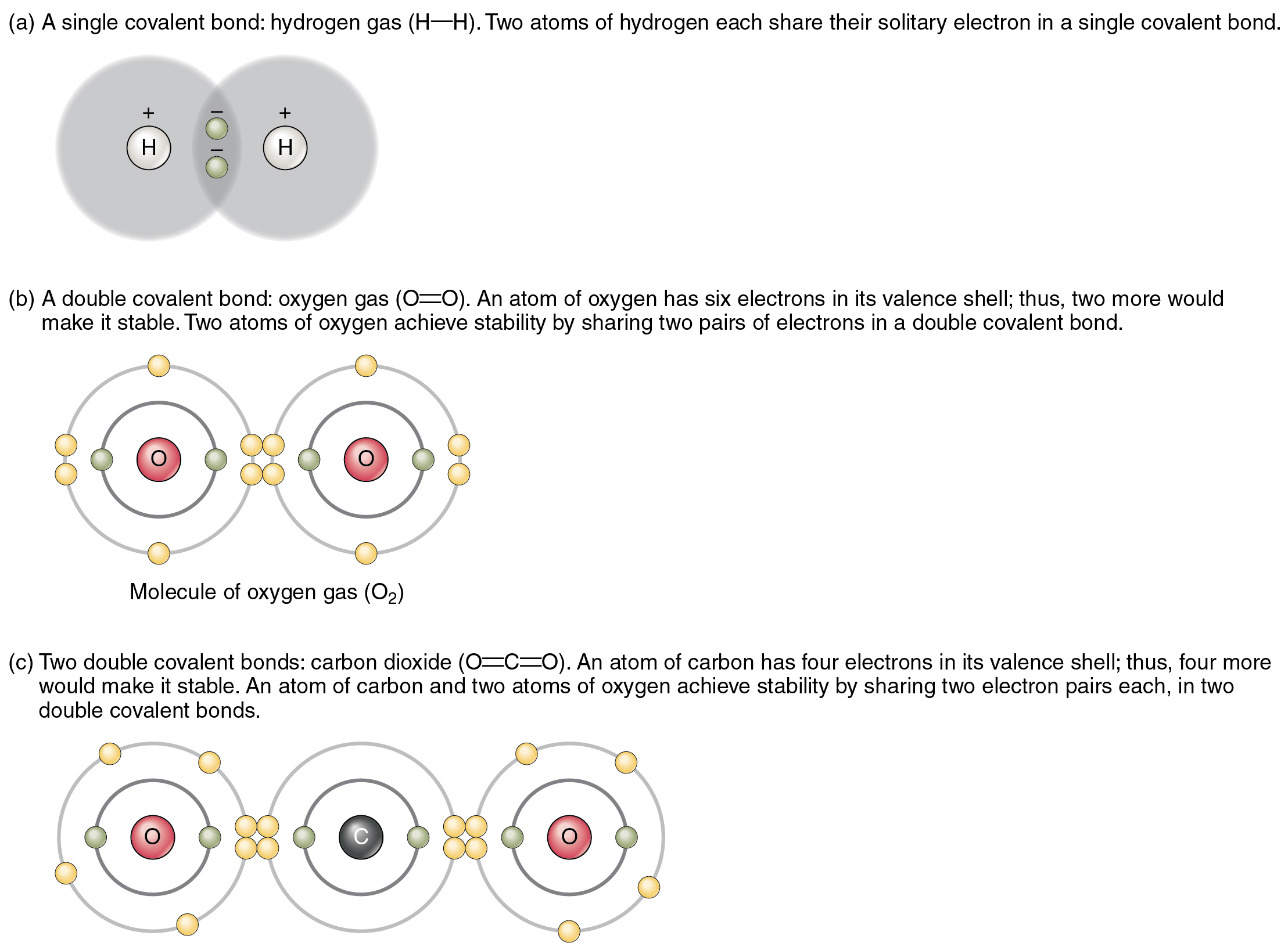
How Is Hydrogen Bonded To Oxygen In Water . the structure of water molecules and how they can interact to form hydrogen bonds. explain how water molecules link via hydrogen bonds. Rather, they must come close enough for the. In h 2 o, only two of the six. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. Atoms separated by a great distance cannot link;
from philschatz.com
in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Atoms separated by a great distance cannot link; the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. the structure of water molecules and how they can interact to form hydrogen bonds. In h 2 o, only two of the six. explain how water molecules link via hydrogen bonds. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Rather, they must come close enough for the.
Chemical Bonds · Anatomy and Physiology
How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. the structure of water molecules and how they can interact to form hydrogen bonds. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Atoms separated by a great distance cannot link; Rather, they must come close enough for the. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. In h 2 o, only two of the six. explain how water molecules link via hydrogen bonds. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183 How Is Hydrogen Bonded To Oxygen In Water the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. In h 2 o, only two of the six. Rather, they must come close enough for the. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a. How Is Hydrogen Bonded To Oxygen In Water.
From www.vrogue.co
Formation Of Hydrogen Bonds In Water Easybiologyclass vrogue.co How Is Hydrogen Bonded To Oxygen In Water the structure of water molecules and how they can interact to form hydrogen bonds. explain how water molecules link via hydrogen bonds. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. in water, each hydrogen nucleus is covalently bound. How Is Hydrogen Bonded To Oxygen In Water.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii How Is Hydrogen Bonded To Oxygen In Water the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Atoms separated by a great distance cannot link; In h 2 o, only two of the six. Rather, they must come close enough for the. in water, each hydrogen nucleus is covalently bound to the central oxygen atom. How Is Hydrogen Bonded To Oxygen In Water.
From drmchemistrytutor.com
Hydrogen Bonding in water Dr. M. Chemistry Tutor How Is Hydrogen Bonded To Oxygen In Water in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Atoms separated by a great distance cannot link;. How Is Hydrogen Bonded To Oxygen In Water.
From www.alamy.com
electrolysis cell of water to produce hydrogen and oxygen Stock Photo How Is Hydrogen Bonded To Oxygen In Water Atoms separated by a great distance cannot link; Rather, they must come close enough for the. the structure of water molecules and how they can interact to form hydrogen bonds. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. explain how water molecules link via hydrogen. How Is Hydrogen Bonded To Oxygen In Water.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight How Is Hydrogen Bonded To Oxygen In Water Atoms separated by a great distance cannot link; the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. the structure of water molecules and how they can interact to form hydrogen bonds. Rather, they must come close enough for the. in water, each hydrogen nucleus is covalently. How Is Hydrogen Bonded To Oxygen In Water.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Water and pH How Is Hydrogen Bonded To Oxygen In Water In h 2 o, only two of the six. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. explain how water molecules link via hydrogen bonds. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a. How Is Hydrogen Bonded To Oxygen In Water.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer How Is Hydrogen Bonded To Oxygen In Water in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. the hydrogen bonds in water allow water. How Is Hydrogen Bonded To Oxygen In Water.
From www.dreamstime.com
Bonding Structure of Water Molecules Hydrogen and Oxygen Stock How Is Hydrogen Bonded To Oxygen In Water the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. In h 2 o, only two of the six. Rather, they must come close enough for the. explain how water molecules link via hydrogen bonds. Atoms separated by a great distance cannot. How Is Hydrogen Bonded To Oxygen In Water.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills How Is Hydrogen Bonded To Oxygen In Water the structure of water molecules and how they can interact to form hydrogen bonds. explain how water molecules link via hydrogen bonds. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. the hydrogen bonds in water allow water to absorb heat by. How Is Hydrogen Bonded To Oxygen In Water.
From www.vecteezy.com
Electrolysis of water forming Hydrogen and Oxygen vector illustration How Is Hydrogen Bonded To Oxygen In Water Atoms separated by a great distance cannot link; in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Rather, they must come close enough for the. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj. How Is Hydrogen Bonded To Oxygen In Water.
From www.vectorstock.com
Electrolysis water forming hydrogen and oxygen Vector Image How Is Hydrogen Bonded To Oxygen In Water in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. Atoms separated by a great distance cannot link; the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. explain how. How Is Hydrogen Bonded To Oxygen In Water.
From sciencenotes.org
Hydrogen Bond Definition and Examples How Is Hydrogen Bonded To Oxygen In Water Atoms separated by a great distance cannot link; the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. Rather, they must come close enough for the. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water. How Is Hydrogen Bonded To Oxygen In Water.
From mungfali.com
Hydrogen Bonding Diagram How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. the structure of water molecules and how they can interact to form hydrogen bonds. In h 2 o, only two of the six. Rather, they must come close enough for the. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145. How Is Hydrogen Bonded To Oxygen In Water.
From www.slideserve.com
PPT hydrogen bond PowerPoint Presentation, free download ID4524678 How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. In h 2 o, only two of the six. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of. How Is Hydrogen Bonded To Oxygen In Water.
From stock.adobe.com
Water molecule structure 3d model, polar compound Two How Is Hydrogen Bonded To Oxygen In Water in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. In h 2 o, only two of the six. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally). How Is Hydrogen Bonded To Oxygen In Water.
From www.dreamstime.com
Water Molecule. Oxygen and Hydrogen Stock Vector Illustration of How Is Hydrogen Bonded To Oxygen In Water in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. explain how water molecules link via hydrogen bonds. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving. How Is Hydrogen Bonded To Oxygen In Water.
From www.pinterest.com
illustration of biochemistry, Water molecule consists of two hydrogen How Is Hydrogen Bonded To Oxygen In Water the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. the structure of water molecules and how they can interact to form hydrogen bonds. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair. How Is Hydrogen Bonded To Oxygen In Water.
From www.alamy.com
Reaction of Hydrogen and Oxygen in New compounds. Water molecule that How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. In h 2 o, only two of the six. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Atoms separated by a great distance cannot link; in water, each hydrogen nucleus is covalently bound to the central oxygen. How Is Hydrogen Bonded To Oxygen In Water.
From www.sliderbase.com
Properties of Water Presentation Biology How Is Hydrogen Bonded To Oxygen In Water Rather, they must come close enough for the. In h 2 o, only two of the six. Atoms separated by a great distance cannot link; in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. the structure of water molecules and. How Is Hydrogen Bonded To Oxygen In Water.
From stock.adobe.com
Chemical reaction model of creating new compounds. Hydrogen and Oxygen How Is Hydrogen Bonded To Oxygen In Water the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. In h 2 o, only two of the six. Rather, they must come close enough for the. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared. How Is Hydrogen Bonded To Oxygen In Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID How Is Hydrogen Bonded To Oxygen In Water Rather, they must come close enough for the. the structure of water molecules and how they can interact to form hydrogen bonds. Atoms separated by a great distance cannot link; in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. explain how water molecules. How Is Hydrogen Bonded To Oxygen In Water.
From www.slideserve.com
PPT This is a water molecule 1 oxygen atom and 2 hydrogen atoms How Is Hydrogen Bonded To Oxygen In Water Rather, they must come close enough for the. explain how water molecules link via hydrogen bonds. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. In h 2 o, only two of the six. in water, each hydrogen nucleus. How Is Hydrogen Bonded To Oxygen In Water.
From www.youtube.com
Hydrogen Bonds In Water Explained Intermolecular Forces YouTube How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. the structure of water molecules and how they can interact to form hydrogen bonds. Atoms separated by a great distance cannot link; in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. in water's hydrogen. How Is Hydrogen Bonded To Oxygen In Water.
From www.alamy.com
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy How Is Hydrogen Bonded To Oxygen In Water in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. the structure of water molecules and how they can interact. How Is Hydrogen Bonded To Oxygen In Water.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Is Hydrogen Bonded To Oxygen In Water In h 2 o, only two of the six. explain how water molecules link via hydrogen bonds. Rather, they must come close enough for the. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. the hydrogen atoms are bound to. How Is Hydrogen Bonded To Oxygen In Water.
From mavink.com
Hydrogen Bond Between Two Water Molecules How Is Hydrogen Bonded To Oxygen In Water in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. the structure of water molecules and how they can interact to form hydrogen bonds. In h 2 o, only two of the six. Atoms separated by a great distance cannot link; the hydrogen bonds. How Is Hydrogen Bonded To Oxygen In Water.
From slideplayer.com
Properties of Water Vocab ppt download How Is Hydrogen Bonded To Oxygen In Water explain how water molecules link via hydrogen bonds. the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. In h 2. How Is Hydrogen Bonded To Oxygen In Water.
From brainly.com
Explain how the model you developed shows that when oxygen combines How Is Hydrogen Bonded To Oxygen In Water the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. In h 2 o, only two of the six. Atoms separated by a great distance cannot link; the structure of water molecules and how they can interact to form hydrogen bonds. . How Is Hydrogen Bonded To Oxygen In Water.
From en.wikipedia.org
Hydrogen bond Wikipedia How Is Hydrogen Bonded To Oxygen In Water the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Atoms separated by a great distance cannot link; explain how water molecules link via hydrogen bonds. the structure of water molecules and how they can interact to form hydrogen bonds. In h 2 o, only two of. How Is Hydrogen Bonded To Oxygen In Water.
From www.researchgate.net
Diagram of hydrogen bonding between two water molecules Download How Is Hydrogen Bonded To Oxygen In Water In h 2 o, only two of the six. Rather, they must come close enough for the. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons,. Atoms separated by a great distance cannot link; in water, each hydrogen nucleus is covalently bound to the central oxygen atom. How Is Hydrogen Bonded To Oxygen In Water.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Is Hydrogen Bonded To Oxygen In Water Atoms separated by a great distance cannot link; the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. In h 2 o, only two of the six. the structure of water molecules and how they can interact to form hydrogen bonds. . How Is Hydrogen Bonded To Oxygen In Water.
From www.britannica.com
water Definition, Chemical Formula, Structure, Molecule, & Facts How Is Hydrogen Bonded To Oxygen In Water the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat. in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Atoms separated by a great distance cannot link; Rather,. How Is Hydrogen Bonded To Oxygen In Water.
From ar.inspiredpencil.com
Water Molecules Hydrogen Bonds How Is Hydrogen Bonded To Oxygen In Water the structure of water molecules and how they can interact to form hydrogen bonds. in water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kj ˣ mol −1 [350]) but has (optimally) an. the hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone. How Is Hydrogen Bonded To Oxygen In Water.
From mavink.com
Hydrogen Oxygen Water Equation How Is Hydrogen Bonded To Oxygen In Water in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Atoms separated by a great distance cannot link; explain how water molecules link via hydrogen bonds. Rather, they must come close enough for the. the structure of water molecules and how they can interact. How Is Hydrogen Bonded To Oxygen In Water.