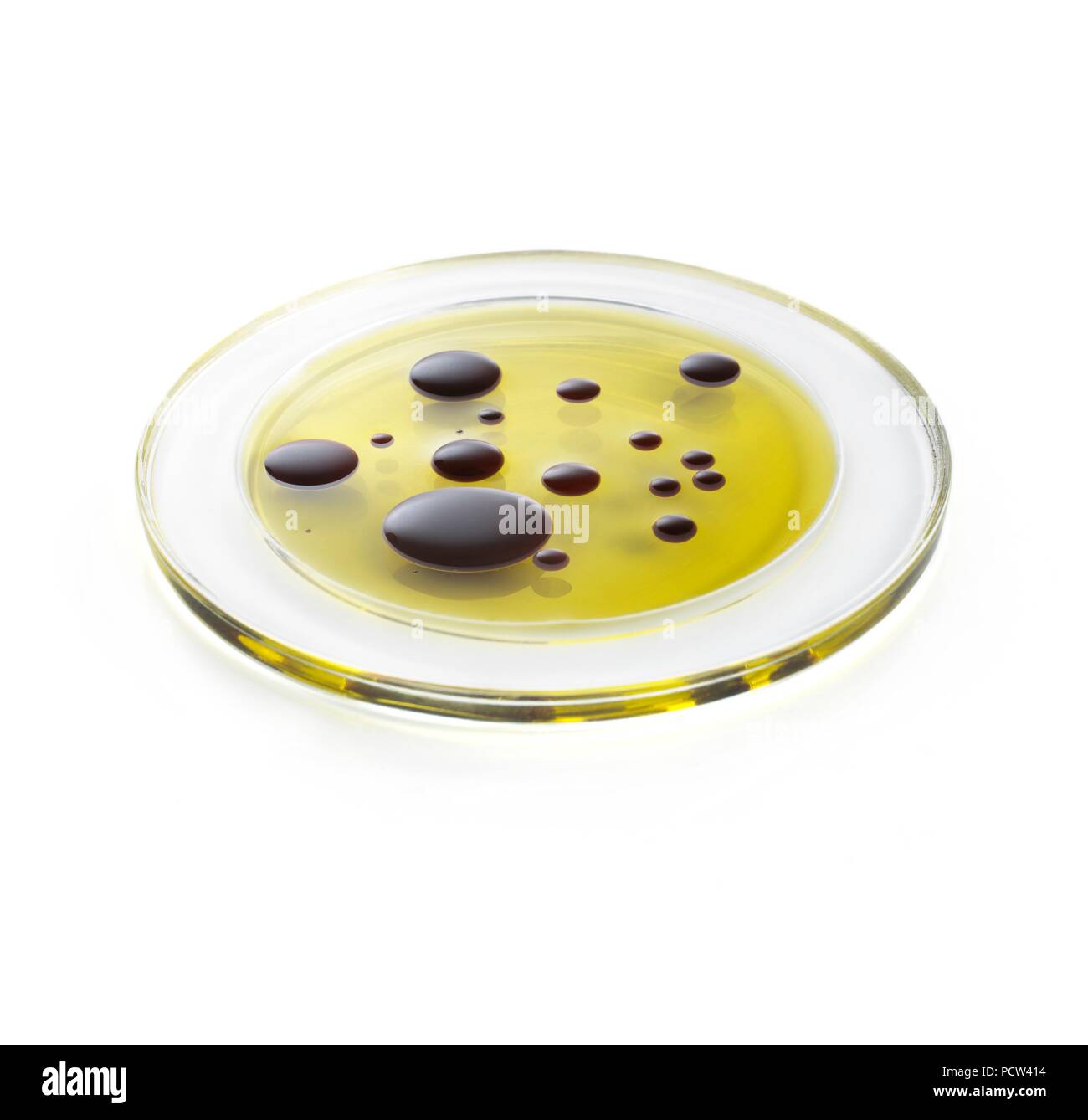
Oil And Vinegar Miscible Or Immiscible . Oil and vinegar are immiscible because they have different polarities. Oil is nonpolar, while vinegar is polar. The same liquid can be miscible with one. Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Find out how surfactants and emulsifiers can make oil and water. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Find out the science behind oil. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution.
from www.alamy.com
Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Find out how surfactants and emulsifiers can make oil and water. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. The same liquid can be miscible with one. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Oil and vinegar are immiscible because they have different polarities. Oil is nonpolar, while vinegar is polar. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not
Oil and vinegar are immiscible hires stock photography and images Alamy
Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible because they have different polarities. Oil and vinegar are immiscible because they have different polarities. Find out how surfactants and emulsifiers can make oil and water. Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. The same liquid can be miscible with one. Find out the science behind oil. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Oil is nonpolar, while vinegar is polar. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers.
From www.alamy.com
An illustration of immiscible liquids oil and water mixed together in Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible because they have different polarities. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. The same liquid can be miscible with one. Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Find out the science behind oil. Learn. Oil And Vinegar Miscible Or Immiscible.
From www.sciencephoto.com
Immiscible liquids Stock Image C014/6055 Science Photo Library Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not A liquid. Oil And Vinegar Miscible Or Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Oil And Vinegar Miscible Or Immiscible Find out the science behind oil. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Find out how surfactants and emulsifiers can make oil and water. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Two liquids are either miscible. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Oil And Vinegar Miscible Or Immiscible Find out how surfactants and emulsifiers can make oil and water. The same liquid can be miscible with one. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Learn why oil and. Oil And Vinegar Miscible Or Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Oil And Vinegar Miscible Or Immiscible Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Find out the science behind oil. The same liquid can be miscible with one. Oil and vinegar are immiscible because they have different polarities. Oil and. Oil And Vinegar Miscible Or Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Oil And Vinegar Miscible Or Immiscible The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil is nonpolar, while vinegar is polar. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. The main difference between miscible and. Oil And Vinegar Miscible Or Immiscible.
From www.numerade.com
SOLVED using the concept of bond polarity solubility explain why water Oil And Vinegar Miscible Or Immiscible Oil is nonpolar, while vinegar is polar. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form. Oil And Vinegar Miscible Or Immiscible.
From www.sciencephoto.com
Oil and vinegar, 2 of 2 Stock Image C050/4776 Science Photo Library Oil And Vinegar Miscible Or Immiscible Find out the science behind oil. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Oil is nonpolar, while vinegar is polar. Oil and vinegar are immiscible because they have different polarities. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no. Oil And Vinegar Miscible Or Immiscible.
From slidetodoc.com
Miscible or Immiscible Differences between miscible and immiscible Oil And Vinegar Miscible Or Immiscible Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Oil and vinegar are immiscible because they have different polarities. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Oil is. Oil And Vinegar Miscible Or Immiscible.
From slidetodoc.com
Miscible or Immiscible Differences between miscible and immiscible Oil And Vinegar Miscible Or Immiscible A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. The mixing of oil and vinegar produces a temporary mixture that. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID2438584 Oil And Vinegar Miscible Or Immiscible Find out how surfactants and emulsifiers can make oil and water. Oil and vinegar are immiscible because they have different polarities. Find out the science behind oil. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT Physical Properties PowerPoint Presentation ID841468 Oil And Vinegar Miscible Or Immiscible Find out how surfactants and emulsifiers can make oil and water. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not A liquid is said to be miscible with respect to another liquid. Oil And Vinegar Miscible Or Immiscible.
From sciencenotes.org
Miscible Definition in Chemistry What Is Miscibility? Oil And Vinegar Miscible Or Immiscible Oil is nonpolar, while vinegar is polar. Oil and vinegar are immiscible because they have different polarities. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the. Oil And Vinegar Miscible Or Immiscible.
From slidetodoc.com
Miscible or Immiscible Differences between miscible and immiscible Oil And Vinegar Miscible Or Immiscible The same liquid can be miscible with one. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. The mixing of oil and vinegar. Oil And Vinegar Miscible Or Immiscible.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Oil And Vinegar Miscible Or Immiscible The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and. Oil And Vinegar Miscible Or Immiscible.
From slidetodoc.com
Miscible or Immiscible Differences between miscible and immiscible Oil And Vinegar Miscible Or Immiscible The same liquid can be miscible with one. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. A liquid is said to be. Oil And Vinegar Miscible Or Immiscible.
From www.thoughtco.com
Immiscible Definition and Examples (Chemistry) Oil And Vinegar Miscible Or Immiscible The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Find out the science behind oil. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Find out how surfactants and emulsifiers can make oil and water. A liquid is said to be miscible with respect to. Oil And Vinegar Miscible Or Immiscible.
From www.alamy.com
Oil and vinegar are immiscible hires stock photography and images Alamy Oil And Vinegar Miscible Or Immiscible The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Find out how surfactants and emulsifiers can make oil and water. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids. Oil And Vinegar Miscible Or Immiscible.
From www.alamy.com
An illustration of immiscible liquids water and oil in an emulsion Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Find out how surfactants and emulsifiers can make oil and water. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into. Oil And Vinegar Miscible Or Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Oil And Vinegar Miscible Or Immiscible Find out the science behind oil. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not A liquid is said to be miscible with respect to another liquid when the two liquids mix. Oil And Vinegar Miscible Or Immiscible.
From www.alamy.com
Emulsion. mixture of liquids that are normally immiscible. experiment Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Oil is nonpolar, while vinegar is polar. The main. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT 25 November 2015 PowerPoint Presentation, free download ID9536690 Oil And Vinegar Miscible Or Immiscible Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. The mixing of oil and vinegar produces a temporary mixture that will eventually separate. Oil And Vinegar Miscible Or Immiscible.
From www.thoughtco.com
Immiscible Definition and Examples (Chemistry) Oil And Vinegar Miscible Or Immiscible Find out how surfactants and emulsifiers can make oil and water. Find out the science behind oil. Oil is nonpolar, while vinegar is polar. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do. Oil And Vinegar Miscible Or Immiscible.
From maverick-has-villa.blogspot.com
Explain Difference Between Miscible and Immiscible Liquids Maverick Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible because they have different polarities. The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. The same liquid can be miscible with one. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Two liquids are either miscible and can mix in. Oil And Vinegar Miscible Or Immiscible.
From www.dreamstime.com
Physics. Immiscible Fluids, Oil and Water. 4 of 4 Image Series. Stock Oil And Vinegar Miscible Or Immiscible The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Oil and vinegar are immiscible because. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2175158 Oil And Vinegar Miscible Or Immiscible Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Find out the science behind oil. Oil and vinegar are immiscible because they have different polarities. The main difference between miscible and immiscible liquids is that miscible liquids are liquids. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT Chemistry deals with the properties of matter. PowerPoint Oil And Vinegar Miscible Or Immiscible The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate. Oil And Vinegar Miscible Or Immiscible.
From slideplayer.com
Solution Chemistry Part I Notes 8b ppt download Oil And Vinegar Miscible Or Immiscible Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. The same liquid can be miscible with one. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all. Oil And Vinegar Miscible Or Immiscible.
From www.slideserve.com
PPT CHAPTER 14 PowerPoint Presentation, free download ID1785921 Oil And Vinegar Miscible Or Immiscible The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Oil and vinegar are immiscible. Oil And Vinegar Miscible Or Immiscible.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Miscible; immiscible Oil And Vinegar Miscible Or Immiscible The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to form a single homogeneous phase, whereas immiscible liquids are liquids that do not Find out the science behind oil. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a. Oil And Vinegar Miscible Or Immiscible.
From slideplayer.com
Chapter 13 “Solutions”. ppt download Oil And Vinegar Miscible Or Immiscible The mixing of oil and vinegar produces a temporary mixture that will eventually separate into two layers. The same liquid can be miscible with one. Find out how surfactants and emulsifiers can make oil and water. The main difference between miscible and immiscible liquids is that miscible liquids are liquids that can mix with each other in all proportions to. Oil And Vinegar Miscible Or Immiscible.
From slideplayer.com
Chapter 2 Matter. ppt download Oil And Vinegar Miscible Or Immiscible Oil and vinegar are immiscible because they have different polarities. Oil is nonpolar, while vinegar is polar. A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. Find out the science behind oil. Learn how the chemical nature of oil and water. Oil And Vinegar Miscible Or Immiscible.
From www.dreamstime.com
Physics. Immiscible Fluids, Oil and Water. Series. 1 0f 4. Stock Image Oil And Vinegar Miscible Or Immiscible Learn how the chemical nature of oil and water molecules causes them to be immiscible, or not mix. Two liquids are either miscible and can mix in any proportion, like vinegar and water, or they are immiscible, such as oil and water, which will separate into two distinct. Oil and vinegar are immiscible liquids that cannot form a true solution,. Oil And Vinegar Miscible Or Immiscible.
From slideplayer.com
Chapter 2 Matter. ppt download Oil And Vinegar Miscible Or Immiscible A liquid is said to be miscible with respect to another liquid when the two liquids mix fully and leave no distinct layer between them in the solution. Find out how surfactants and emulsifiers can make oil and water. The same liquid can be miscible with one. The mixing of oil and vinegar produces a temporary mixture that will eventually. Oil And Vinegar Miscible Or Immiscible.
From slidetodoc.com
Miscible or Immiscible Differences between miscible and immiscible Oil And Vinegar Miscible Or Immiscible The same liquid can be miscible with one. Oil and vinegar are immiscible liquids that cannot form a true solution, but they can create a temporary emulsion with an. Oil and vinegar are immiscible because they have different polarities. Learn why oil and vinegar separate and how to emulsify them with proper techniques and emulsifiers. Two liquids are either miscible. Oil And Vinegar Miscible Or Immiscible.