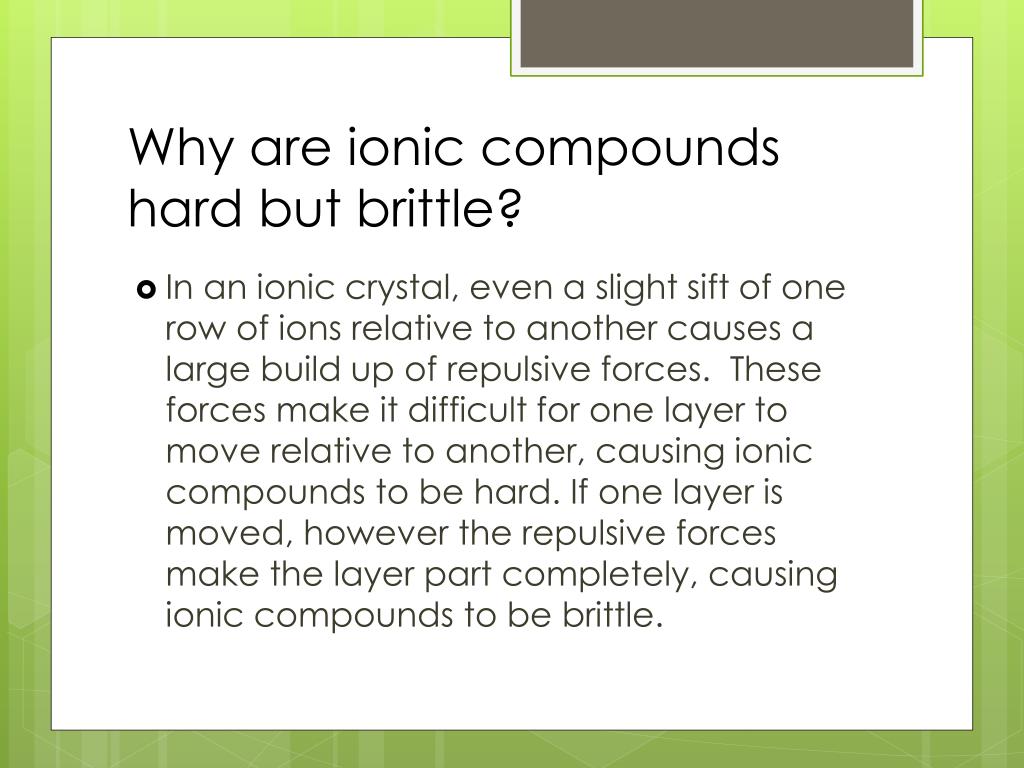
Ionic Compounds Tend To Be Brittle . A hard material will tend to assume its shape when stressed by an external. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ionic compounds are generally hard, but brittle. Ionic solids exhibit a crystalline structure and tend to be. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. The properties of ionic compounds shed some light on the nature of ionic bonds. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds exhibit the property of hardness.
from www.slideserve.com
The properties of ionic compounds shed some light on the nature of ionic bonds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to one another (see below). A hard material will tend to assume its shape when stressed by an external. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't.
PPT Ionic and Covalent bonds PowerPoint Presentation, free download
Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal. The properties of ionic compounds shed some light on the nature of ionic bonds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. The properties of ionic compounds shed some light on the nature of ionic bonds. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are generally hard, but brittle. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds are generally hard, but brittle. A hard material will tend to assume its shape when stressed by an external. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds exhibit the property of hardness. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't.
From slideplayer.com
Ionic Bonding/Naming. ppt download Ionic Compounds Tend To Be Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Ionic Compounds Tend To Be Brittle Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. It takes a large amount of mechanical force, such as striking a crystal. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Ionic Bonding. ppt download Ionic Compounds Tend To Be Brittle Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT Chemical Compounds PowerPoint Presentation, free download ID Ionic Compounds Tend To Be Brittle Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard, but brittle. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds are generally. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 10 Preview Section 1 Ionic and Covalent Compounds ppt download Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are generally hard, but brittle. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic solids exhibit a crystalline structure and tend to be. Ionic solids exhibit a crystalline structure and tend to be. It takes. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Ionic Bonding. ppt download Ionic Compounds Tend To Be Brittle A hard material will tend to assume its shape when stressed by an external. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds tend to be hard and brittle while covalent compounds tend. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Ionic and Covalent Compounds ppt download Ionic Compounds Tend To Be Brittle A hard material will tend to assume its shape when stressed by an external. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds exhibit the property of hardness. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds conduct electricity. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
How to Use This Presentation ppt download Ionic Compounds Tend To Be Brittle Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to one another. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Click a hyperlink to view the corresponding slides. ppt download Ionic Compounds Tend To Be Brittle The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds conduct electricity when melted or dissolved. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Ionic Bonds. ppt download Ionic Compounds Tend To Be Brittle Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. The properties of ionic compounds shed some light on the nature of ionic bonds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However,. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 6 Chemical Bonds ppt download Ionic Compounds Tend To Be Brittle Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. It takes a large amount of mechanical force, such as striking. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Tend To Be Brittle The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds are generally hard, but brittle. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chemical Compounds Chapter 10 Pages ppt download Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds exhibit the property of hardness. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The properties. Ionic Compounds Tend To Be Brittle.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Ionic compounds conduct electricity when melted or dissolved in water. Ionic Compounds Tend To Be Brittle.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Ionic Compounds Tend To Be Brittle Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. Ionic compounds exhibit the property of hardness. Ionic compounds are brittle. Ionic Compounds Tend To Be Brittle.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Ionic Compounds Tend To Be Brittle Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic solids exhibit a crystalline. Ionic Compounds Tend To Be Brittle.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Ionic Compounds Tend To Be Brittle Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are generally hard, but brittle. The properties of ionic compounds shed some light. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Goals for the next 2 days ppt download Ionic Compounds Tend To Be Brittle A hard material will tend to assume its shape when stressed by an external. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are generally hard, but brittle. Ionic compounds conduct electricity when melted or dissolved in. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Mr. Conkey Physical Science Ch ppt download Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal. The properties of ionic compounds shed some light on the nature of ionic bonds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic solids exhibit a crystalline. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 6 Section 1 Compounds and Molecules ppt download Ionic Compounds Tend To Be Brittle The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic solids exhibit. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Ionic Compounds Tend To Be Brittle Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. The properties of ionic compounds shed some. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT Chemical Compounds PowerPoint Presentation, free download ID Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. A hard material will tend to assume its shape when stressed by an external. Ionic solids exhibit a crystalline structure and tend to be. However, when that happens, it brings ions of. Ionic Compounds Tend To Be Brittle.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Ionic Compounds Tend To Be Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be. Ionic. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Polyatomic Ions. ppt download Ionic Compounds Tend To Be Brittle Ionic compounds exhibit the property of hardness. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic solids exhibit a crystalline structure and tend to be. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds are brittle due to the strong bond between the. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Ionic Compounds Tend To Be Brittle However, when that happens, it brings ions of the same charge next to one another (see below). The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds are brittle due to the strong bond between the. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 6 Section 1 Compounds and Molecules ppt download Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. The properties of ionic compounds shed some light on the nature of ionic bonds. However, when that happens, it brings ions of the same charge next to one another (see below). Ionic. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Structure of Atoms. ppt download Ionic Compounds Tend To Be Brittle Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. A hard material will tend. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Tend To Be Brittle Ionic compounds exhibit the property of hardness. However, when that happens, it brings ions of the same charge next to one another (see below). A hard material will tend to assume its shape when stressed by an external. Ionic solids exhibit a crystalline structure and tend to be. The properties of ionic compounds shed some light on the nature of. Ionic Compounds Tend To Be Brittle.
From www.ck12.org
Ionic Compounds 1 CK12 Foundation Ionic Compounds Tend To Be Brittle However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. It takes a large amount of mechanical force, such as striking a crystal. The properties of ionic compounds shed some light on the nature of. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Bell Work. Ionic and Covalent Compounds Ions and molecules can combine Ionic Compounds Tend To Be Brittle Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds exhibit the property of hardness. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 6 Ionic and Covalent Bonds and Formulas. In our last unit… ATOM Ionic Compounds Tend To Be Brittle However, when that happens, it brings ions of the same charge next to one another (see below). Ionic compounds are generally hard, but brittle. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic solids exhibit a crystalline structure and tend to be. Ionic compounds exhibit the property of hardness. Ionic. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 1 Atoms and Elements ppt download Ionic Compounds Tend To Be Brittle A hard material will tend to assume its shape when stressed by an external. Ionic solids exhibit a crystalline structure and tend to be. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds conduct electricity when melted or dissolved. Ionic Compounds Tend To Be Brittle.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Ionic Compounds Tend To Be Brittle Ionic compounds conduct electricity when melted or dissolved in water while covalent compounds typically don't. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds tend to be hard and brittle while covalent. Ionic Compounds Tend To Be Brittle.
From www.slideshare.net
Ionic Bonding Notes Ionic Compounds Tend To Be Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard, but brittle. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a. Ionic Compounds Tend To Be Brittle.
From slideplayer.com
Chapter 9 Models of Chemical Bonding. ppt download Ionic Compounds Tend To Be Brittle Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Ionic compounds exhibit the property of hardness. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. Ionic compounds are generally hard, but brittle.. Ionic Compounds Tend To Be Brittle.