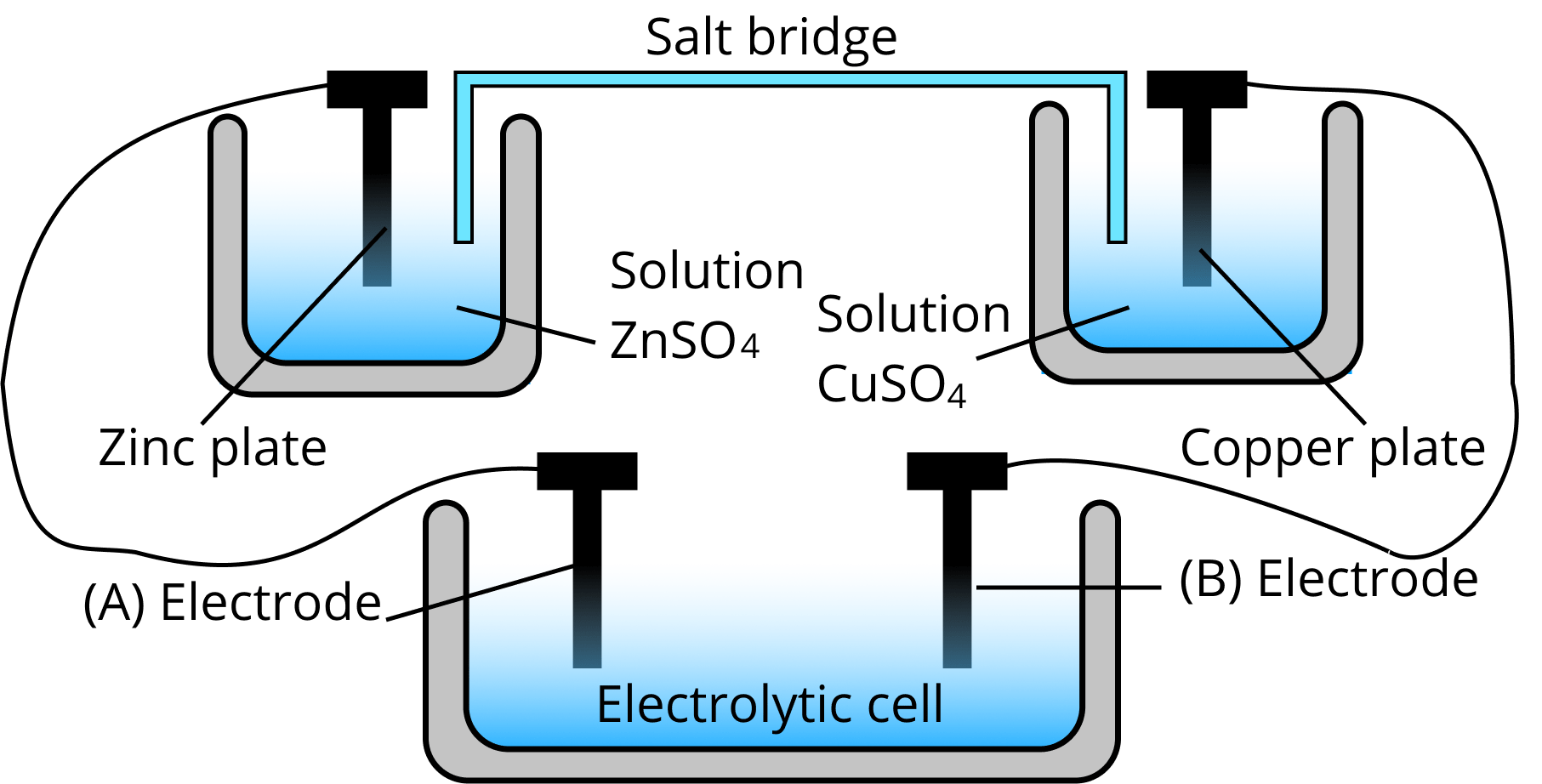
Salt Bridge Explanation . The circuit is closed using a salt bridge, which transmits the current with moving ions. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak.
from www.vedantu.com
In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge is a vital component of any voltaic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. The circuit is closed using a salt bridge, which transmits the current with moving ions. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak.
Electrochemistry Class 12 Notes for NEET Chemistry Revision
Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. The circuit is closed using a salt bridge, which transmits the current with moving ions. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. The salt bridge is a vital component of any voltaic cell. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another.
From www.youtube.com
KAC32.17 Electrochemistry The Role of the Salt Bridge YouTube Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. A salt bridge is a type of laboratory device used. Salt Bridge Explanation.
From www.youtube.com
Salt bridge What is Salt Brigde Application of salt bridge Salt Bridge Explanation In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a. Salt Bridge Explanation.
From tyreseghopsweeney.blogspot.com
What Does a Salt Bridge Do Apex Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. A salt bridge is a type of laboratory device used in an. Salt Bridge Explanation.
From www.youtube.com
Functions of Salt bridge part 7 electro chemistry CBSE chemistry Salt Bridge Explanation It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The circuit is closed using a salt bridge, which transmits the current with moving ions. The salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3. Salt Bridge Explanation.
From www.thoughtco.com
What Is a Salt Bridge? Salt Bridge Explanation The salt bridge is a vital component of any voltaic cell. The circuit is closed using a salt bridge, which transmits the current with moving ions. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction. Salt Bridge Explanation.
From www.youtube.com
Salt bridge (Electrochemistry part 9 for CBSE class 12 JEE IIT) YouTube Salt Bridge Explanation The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation. Salt Bridge Explanation.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID2275817 Salt Bridge Explanation The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. In other words, a salt bridge is a junction that connects the. Salt Bridge Explanation.
From brainly.in
Salt bridge diagram and explanation Brainly.in Salt Bridge Explanation In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. The circuit is closed using a salt bridge, which transmits the current with. Salt Bridge Explanation.
From study.com
Quiz & Worksheet Salt Bridge Description Salt Bridge Explanation In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. The salt bridge is a vital component of any voltaic cell. A salt bridge is. Salt Bridge Explanation.
From www.slideserve.com
PPT Natural Polymers PowerPoint Presentation, free download ID2609255 Salt Bridge Explanation A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. The salt bridge is a vital component of any voltaic cell. A. Salt Bridge Explanation.
From www.youtube.com
FUNCTION OF SALT BRIDGE (Chemistry Form 5) YouTube Salt Bridge Explanation It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The circuit is closed using a salt bridge, which transmits the current with moving ions. The purpose of. Salt Bridge Explanation.
From www.youtube.com
Salt bridges in proteins YouTube Salt Bridge Explanation The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions. Salt Bridge Explanation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4491325 Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. The salt bridge is a vital component of any voltaic. Salt Bridge Explanation.
From brainly.in
what is the function of salt bridge ? Brainly.in Salt Bridge Explanation A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The salt bridge consists of a concentrated, nonreactive, electrolyte solution. Salt Bridge Explanation.
From www.youtube.com
FUNCTION OF SALT BRIDGE YouTube Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. The purpose of the salt bridge is to keep the. Salt Bridge Explanation.
From www.vedantu.com
Electrochemistry Class 12 Notes for NEET Chemistry Revision Salt Bridge Explanation The circuit is closed using a salt bridge, which transmits the current with moving ions. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or. Salt Bridge Explanation.
From www.researchgate.net
Salt bridge bonds between IMM01 and CD47. Download Scientific Diagram Salt Bridge Explanation The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. It is a tube filled with an electrolyte solution such as kno. Salt Bridge Explanation.
From www.researchgate.net
Analysis of salt bridges by MD simulations. Panel A, Identification of Salt Bridge Explanation The circuit is closed using a salt bridge, which transmits the current with moving ions. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. The salt bridge is a vital component of any voltaic cell. In other words, a salt bridge is a junction that connects. Salt Bridge Explanation.
From www.researchgate.net
Formation of salt bridges in different umbrella sampling windows. Left Salt Bridge Explanation In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. It is a tube filled with an electrolyte solution such as kno 3. Salt Bridge Explanation.
From www.pinterest.com
Salt Bridge definition A tube containing a strong electrolyte used to Salt Bridge Explanation The circuit is closed using a salt bridge, which transmits the current with moving ions. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The salt bridge consists of a. Salt Bridge Explanation.
From www.researchgate.net
Salt bridges in the N and C termini of highalkaline Mprotease Salt Bridge Explanation The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge is a vital component of any voltaic cell. The purpose of the salt bridge is to keep. Salt Bridge Explanation.
From www.researchgate.net
Saltbridge interactions at the dimer interface are partially important Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The salt bridge consists of a concentrated, nonreactive, electrolyte solution. Salt Bridge Explanation.
From www.youtube.com
5 Electrochemistry How Salt Bridge Works? Movement of ions in Salt Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The salt bridge is a vital component of any voltaic cell. A salt. Salt Bridge Explanation.
From www.youtube.com
What is salt Bridge & state its function Electrochemistry Physical Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. A salt bridge is a device used in an electrochemical. Salt Bridge Explanation.
From www.numerade.com
⏩SOLVEDExplain the purpose of a salt bridge in an electrochemical Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge is a vital component of any voltaic cell. In other words, a salt bridge is. Salt Bridge Explanation.
From www.meritnation.com
what is salt bridge,explain its functions Chemistry Redox Reactions Salt Bridge Explanation It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow. Salt Bridge Explanation.
From www.researchgate.net
Flowchart of saltbridge design in this study. Identification of Salt Bridge Explanation It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or. Salt Bridge Explanation.
From www.youtube.com
How the salt bridge works YouTube Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The salt bridge is a vital component of any voltaic cell. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The purpose. Salt Bridge Explanation.
From www.researchgate.net
1 The structure of a salt bridge formed between aspartate and lysine Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the. Salt Bridge Explanation.
From jamarion-has-cuevas.blogspot.com
What Is the Purpose of a Salt Bridge JamarionhasCuevas Salt Bridge Explanation It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction. Salt Bridge Explanation.
From www.youtube.com
Salt Bridge YouTube Salt Bridge Explanation The circuit is closed using a salt bridge, which transmits the current with moving ions. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3 ) solution used in this example. A salt bridge is a. Salt Bridge Explanation.
From achs-prod.acs.org
A Lateral Salt Bridge for the Specific Assembly of an ABCType Collagen Salt Bridge Explanation The circuit is closed using a salt bridge, which transmits the current with moving ions. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or. Salt Bridge Explanation.
From www.youtube.com
Salt bridge YouTube Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. In other words, a salt bridge is a junction that. Salt Bridge Explanation.
From www.youtube.com
Describe the function of a salt bridge. YouTube Salt Bridge Explanation The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half. Salt Bridge Explanation.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Salt Bridge Explanation A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. A salt bridge is a type of laboratory device used in an electrochemical cell to connect its reduction and oxidation half cells wherein a weak. The salt bridge consists of a concentrated, nonreactive, electrolyte solution. Salt Bridge Explanation.