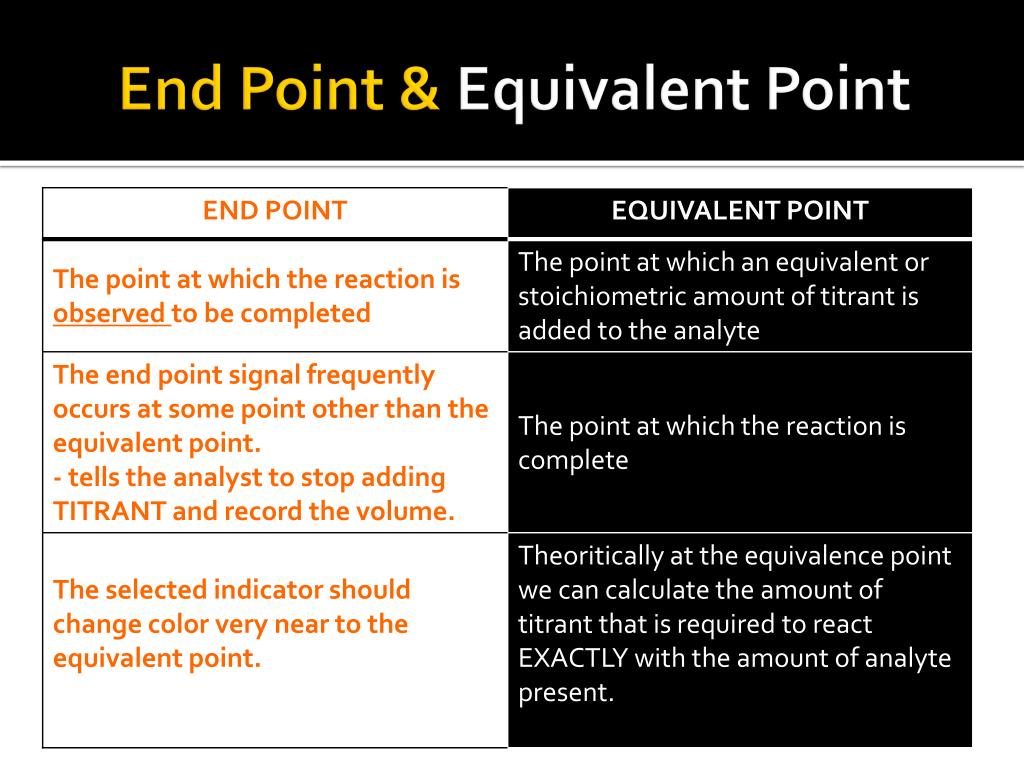
Titrant Endpoint Vs Equivalence Point . The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. There are many different types of titrations that differ by the titrant used and substances that can be. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In contrast, the equivalence point represents. Definitions of titrant, titration curve, end point and equivalence point.
from www.slideserve.com
Definitions of titrant, titration curve, end point and equivalence point. In contrast, the equivalence point represents. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). There are many different types of titrations that differ by the titrant used and substances that can be. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the.
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free
Titrant Endpoint Vs Equivalence Point In contrast, the equivalence point represents. In contrast, the equivalence point represents. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). There are many different types of titrations that differ by the titrant used and substances that can be. Definitions of titrant, titration curve, end point and equivalence point. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,.
From giosstgvo.blob.core.windows.net
Titration Quiz Questions at Kevin Horton blog Titrant Endpoint Vs Equivalence Point In contrast, the equivalence point represents. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. There are many different types of titrations that differ by the titrant used and substances that can be. The difference between the endpoint and equivalence point is that an observable color change in. Titrant Endpoint Vs Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titrant Endpoint Vs Equivalence Point The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte).. Titrant Endpoint Vs Equivalence Point.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Titrant Endpoint Vs Equivalence Point There are many different types of titrations that differ by the titrant used and substances that can be. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. In contrast, the equivalence point represents. The main difference between equivalence and endpoint is that the equivalence. Titrant Endpoint Vs Equivalence Point.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF Titrant Endpoint Vs Equivalence Point Definitions of titrant, titration curve, end point and equivalence point. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The main difference between equivalence and endpoint is that the equivalence. Titrant Endpoint Vs Equivalence Point.
From www.conquerhsc.com
HSC Chemistry Module 6 Inquiry Question 3 Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. There are many different types of titrations that differ by the titrant used and substances that. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. The equivalence point is the point at which titrant has been added in exactly the right. Titrant Endpoint Vs Equivalence Point.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Titrant Endpoint Vs Equivalence Point In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the. Titrant Endpoint Vs Equivalence Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Titrant Endpoint Vs Equivalence Point Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. Definitions of titrant, titration curve, end point and equivalence point. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is. Titrant Endpoint Vs Equivalence Point.
From www.differencebetween.com
Difference Between Endpoint and Stoichiometric Point Compare the Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. In contrast, the equivalence point represents. When it comes to chemical reactions. Titrant Endpoint Vs Equivalence Point.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Titrant Endpoint Vs Equivalence Point In contrast, the equivalence point represents. Definitions of titrant, titration curve, end point and equivalence point. There are many different types of titrations that differ by the titrant used and substances that can be. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The main difference between equivalence and endpoint. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Definitions of titrant, titration curve, end point and equivalence point. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point. Titrant Endpoint Vs Equivalence Point.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Titrant Endpoint Vs Equivalence Point There are many different types of titrations that differ by the titrant used and substances that can be. In contrast, the equivalence point represents. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). When it comes to chemical. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Titrant Endpoint Vs Equivalence Point When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. Definitions of titrant, titration curve, end point and equivalence point. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Based on this ladder diagram, the. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Titrant Endpoint Vs Equivalence Point When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. Definitions of titrant, titration curve, end point and equivalence point. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. In contrast, the equivalence point represents. There are many different. Titrant Endpoint Vs Equivalence Point.
From askanydifference.com
Endpoint vs Equivalence Point Difference and Comparison Titrant Endpoint Vs Equivalence Point The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). There are many different types of titrations that differ by the titrant used and substances that can be. In the titration procedure, a known concentration solution is known as. Titrant Endpoint Vs Equivalence Point.
From www.differencebetween.com
Difference Between Endpoint and Stoichiometric Point Compare the Titrant Endpoint Vs Equivalence Point Definitions of titrant, titration curve, end point and equivalence point. In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. Based on this ladder diagram, the first equivalence point is between. Titrant Endpoint Vs Equivalence Point.
From www.chegg.com
Solved 7. Explain the difference between equivalence point Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. There are many different types of titrations that differ by the titrant used and substances that can be. Definitions of titrant, titration curve, end point and equivalence point. Based on this ladder diagram, the first equivalence point. Titrant Endpoint Vs Equivalence Point.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. The equivalence point is the point at which. Titrant Endpoint Vs Equivalence Point.
From www.vrogue.co
Difference Between Endpoint And Equivalence Point1 Di vrogue.co Titrant Endpoint Vs Equivalence Point When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The main difference between equivalence point and endpoint is that equivalence point is the actual point. Titrant Endpoint Vs Equivalence Point.
From exoxoimsp.blob.core.windows.net
Point Titration Definition at Gerald Lathrop blog Titrant Endpoint Vs Equivalence Point The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. Definitions of titrant, titration curve, end point and equivalence point. There are many different types of titrations that differ by the titrant used and substances that can be. In the titration. Titrant Endpoint Vs Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titrant Endpoint Vs Equivalence Point In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the. Titrant Endpoint Vs Equivalence Point.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Titrant Endpoint Vs Equivalence Point When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. There are many different types of titrations that differ by the titrant used and substances that can be. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to. Titrant Endpoint Vs Equivalence Point.
From www.numerade.com
SOLVED The different between endpoint vs equivalence point which one Titrant Endpoint Vs Equivalence Point Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyten (when moles of titrant = moles of analyte). There are many different. Titrant Endpoint Vs Equivalence Point.
From www.numerade.com
SOLVED Explain the difference between end point and equivalence point Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. Definitions of titrant, titration curve, end point and equivalence point. In contrast, the equivalence point represents. There. Titrant Endpoint Vs Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Titrant Endpoint Vs Equivalence Point Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. When it comes to chemical reactions and titrations, two important terms that often come up are endpoint and equivalence point. these. The main difference between equivalence point and endpoint is that equivalence point is the. Titrant Endpoint Vs Equivalence Point.
From philschatz.com
AcidBase Titrations · Chemistry Titrant Endpoint Vs Equivalence Point Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. In contrast, the equivalence point represents. Definitions of titrant, titration curve, end point and equivalence point. The equivalence point is the point at which titrant has been added in exactly the right quantity to react. Titrant Endpoint Vs Equivalence Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titrant Endpoint Vs Equivalence Point Definitions of titrant, titration curve, end point and equivalence point. The difference between the endpoint and equivalence point is that an observable color change in the indicator determines the endpoint. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In the titration procedure, a known concentration. Titrant Endpoint Vs Equivalence Point.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titrant Endpoint Vs Equivalence Point Definitions of titrant, titration curve, end point and equivalence point. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the. Titrant Endpoint Vs Equivalence Point.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Titrant Endpoint Vs Equivalence Point In the titration procedure, a known concentration solution is known as the titrant, whereas an unknown concentration solution is. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. There are many different types of titrations that differ by the titrant used and substances that can be.. Titrant Endpoint Vs Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titrant Endpoint Vs Equivalence Point The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Definitions of titrant, titration curve, end point and equivalence point. Based on this ladder diagram, the first equivalence point is between a ph of 3.13 and a ph of 4.76, the second equivalence point is. The equivalence. Titrant Endpoint Vs Equivalence Point.