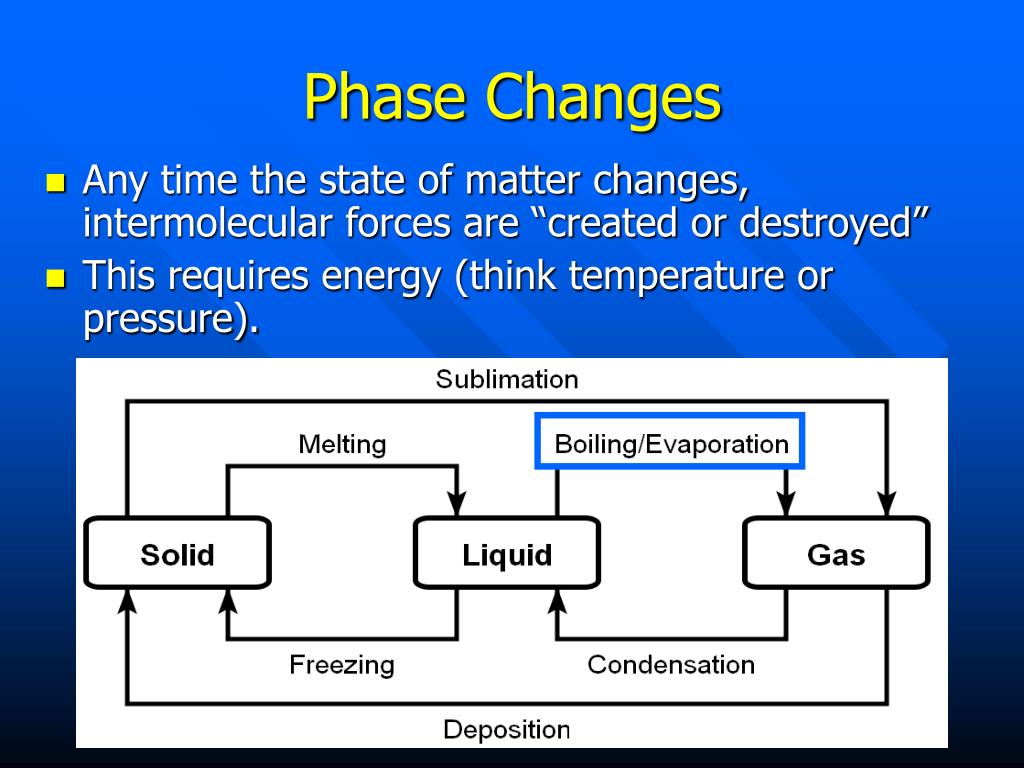
Why Does Pressure Affect Phase Change . A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance. The existence of the three phases with. In the picture above, we have a container. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. the main factors that cause phase changes are changes in temperature and pressure. These changes occur when sufficient energy is. At the phase transition, such as the. describe the relationship between heat (energy), bonding forces, and phase changes.
from www.slideserve.com
Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. The existence of the three phases with. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we have a container. describe the relationship between heat (energy), bonding forces, and phase changes. At the phase transition, such as the. the main factors that cause phase changes are changes in temperature and pressure. These changes occur when sufficient energy is.
PPT Phase Changes PowerPoint Presentation, free download ID5049190
Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. These changes occur when sufficient energy is. the main factors that cause phase changes are changes in temperature and pressure. At the phase transition, such as the. describe the relationship between heat (energy), bonding forces, and phase changes. The existence of the three phases with. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. In the picture above, we have a container. phase changes among the various phases of matter depend on temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. Pressure can also be used to change the phase of the substance.
From courses.lumenlearning.com
Phase Changes Physics Why Does Pressure Affect Phase Change In the picture above, we have a container. These changes occur when sufficient energy is. At the phase transition, such as the. Pressure can also be used to change the phase of the substance. the main factors that cause phase changes are changes in temperature and pressure. A phase change is when matter changes to from one state (solid,. Why Does Pressure Affect Phase Change.
From courses.lumenlearning.com
Phase Changes Physics Why Does Pressure Affect Phase Change Pressure can also be used to change the phase of the substance. phase changes among the various phases of matter depend on temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. The existence of the three phases with. the main factors that cause phase changes are changes. Why Does Pressure Affect Phase Change.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Why Does Pressure Affect Phase Change These changes occur when sufficient energy is. Pressure can also be used to change the phase of the substance. describe the relationship between heat (energy), bonding forces, and phase changes. the main factors that cause phase changes are changes in temperature and pressure. phase changes among the various phases of matter depend on temperature and pressure. The. Why Does Pressure Affect Phase Change.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Reactions MCAT Content Why Does Pressure Affect Phase Change describe the relationship between heat (energy), bonding forces, and phase changes. These changes occur when sufficient energy is. At the phase transition, such as the. phase changes among the various phases of matter depend on temperature and pressure. the main factors that cause phase changes are changes in temperature and pressure. A phase change is when matter. Why Does Pressure Affect Phase Change.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Why Does Pressure Affect Phase Change note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. The existence of the three phases with. describe the relationship between heat (energy), bonding forces, and phase changes. phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we. Why Does Pressure Affect Phase Change.
From unistudium.unipg.it
Phase Diagrams Why Does Pressure Affect Phase Change the main factors that cause phase changes are changes in temperature and pressure. Pressure can also be used to change the phase of the substance. describe the relationship between heat (energy), bonding forces, and phase changes. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure.. Why Does Pressure Affect Phase Change.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Reactions MCAT Content Why Does Pressure Affect Phase Change In the picture above, we have a container. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. phase changes among the various phases of matter depend on temperature and pressure. the main factors that cause phase changes are changes in temperature and pressure. describe. Why Does Pressure Affect Phase Change.
From www.youtube.com
What is the effect of pressure change on equilibrium Class 10 Chemistry Chemical Equilibrium Why Does Pressure Affect Phase Change The existence of the three phases with. Pressure can also be used to change the phase of the substance. the main factors that cause phase changes are changes in temperature and pressure. At the phase transition, such as the. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. describe the. Why Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Phase Diagrams PowerPoint Presentation, free download ID704549 Why Does Pressure Affect Phase Change At the phase transition, such as the. The existence of the three phases with. These changes occur when sufficient energy is. the main factors that cause phase changes are changes in temperature and pressure. phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we have a container. A phase change. Why Does Pressure Affect Phase Change.
From www.youtube.com
Effect of pressure Change of state from gas to liquid CBSE Class 9 Science YouTube Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. At the phase transition, such as the. the main factors that cause phase changes are changes in temperature and pressure. describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of the substance. . Why Does Pressure Affect Phase Change.
From manualfixporterly55.z22.web.core.windows.net
How To Read Phase Diagrams Chemistry Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. the main factors that cause phase changes are changes in temperature and pressure. note that, at a fixed temperature, you can change the phase from solid (ice). Why Does Pressure Affect Phase Change.
From lessonlibcordwainer.z22.web.core.windows.net
How Does Temperature Affect Phase Changes Why Does Pressure Affect Phase Change note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. These changes occur when sufficient energy is. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. the main factors that cause phase changes are changes in temperature and pressure.. Why Does Pressure Affect Phase Change.
From philschatz.com
Phase Changes · Physics Why Does Pressure Affect Phase Change These changes occur when sufficient energy is. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. Pressure can also be used to change the phase of the substance. describe the relationship between heat (energy), bonding forces, and phase changes. In the picture above, we have a container. the main factors. Why Does Pressure Affect Phase Change.
From pressbooks.bccampus.ca
Phase Changes Basic HVAC Why Does Pressure Affect Phase Change The existence of the three phases with. At the phase transition, such as the. describe the relationship between heat (energy), bonding forces, and phase changes. the main factors that cause phase changes are changes in temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that,. Why Does Pressure Affect Phase Change.
From socratic.org
What is the relation between critical temperature and boiling point or vapor pressure? Socratic Why Does Pressure Affect Phase Change At the phase transition, such as the. phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we have a container. These changes occur when sufficient energy is. The existence of the three phases with. describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used. Why Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with. At the phase transition, such as the. describe the relationship between heat (energy), bonding forces, and phase changes. These changes occur when sufficient energy is. the main factors that cause phase changes are changes in temperature and. Why Does Pressure Affect Phase Change.
From ceuulqzk.blob.core.windows.net
Why Does Pressure Change With Temperature at Brenda Marx blog Why Does Pressure Affect Phase Change note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. The existence of the three phases with. These changes occur when sufficient energy is. In the picture above, we have a container. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to. Why Does Pressure Affect Phase Change.
From printableseniena9s.z21.web.core.windows.net
Heat Of Phase Change Why Does Pressure Affect Phase Change In the picture above, we have a container. These changes occur when sufficient energy is. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. At the phase transition, such as the. phase changes among the various phases of matter depend on temperature and pressure. Pressure can. Why Does Pressure Affect Phase Change.
From ceuulqzk.blob.core.windows.net
Why Does Pressure Change With Temperature at Brenda Marx blog Why Does Pressure Affect Phase Change the main factors that cause phase changes are changes in temperature and pressure. phase changes among the various phases of matter depend on temperature and pressure. At the phase transition, such as the. Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change the phase from. Why Does Pressure Affect Phase Change.
From schematicheelazoonrj.z4.web.core.windows.net
How To Interpret A Phase Diagram Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. Pressure can also be used to change the phase of the substance. These changes occur when sufficient energy is. In the picture above, we have a container. At the. Why Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Solutions PowerPoint Presentation ID6014910 Why Does Pressure Affect Phase Change A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. The existence of the three phases with. phase changes among the various phases of matter depend on temperature and pressure.. Why Does Pressure Affect Phase Change.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Why Does Pressure Affect Phase Change the main factors that cause phase changes are changes in temperature and pressure. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. These changes occur when sufficient energy is. describe the relationship between heat (energy), bonding forces, and phase changes. At the phase transition, such. Why Does Pressure Affect Phase Change.
From www.ck12.org
Phase Diagrams CK12 Foundation Why Does Pressure Affect Phase Change The existence of the three phases with. phase changes among the various phases of matter depend on temperature and pressure. These changes occur when sufficient energy is. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a container. Pressure can also be used to change. Why Does Pressure Affect Phase Change.
From ceuulqzk.blob.core.windows.net
Why Does Pressure Change With Temperature at Brenda Marx blog Why Does Pressure Affect Phase Change The existence of the three phases with. At the phase transition, such as the. Pressure can also be used to change the phase of the substance. phase changes among the various phases of matter depend on temperature and pressure. the main factors that cause phase changes are changes in temperature and pressure. note that, at a fixed. Why Does Pressure Affect Phase Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Why Does Pressure Affect Phase Change The existence of the three phases with. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. At the phase transition, such as the. In the picture above, we have a. Why Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3571033 Why Does Pressure Affect Phase Change note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. These changes occur when sufficient energy is. In the picture above, we have a container. the main factors that cause. Why Does Pressure Affect Phase Change.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Why Does Pressure Affect Phase Change A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a container. the main factors that cause phase changes are changes in temperature and pressure. Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can. Why Does Pressure Affect Phase Change.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with. describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of the substance. At the phase transition, such as the. In the picture above, we have a container.. Why Does Pressure Affect Phase Change.
From nursehub.com
Phase Changes NurseHub Why Does Pressure Affect Phase Change describe the relationship between heat (energy), bonding forces, and phase changes. phase changes among the various phases of matter depend on temperature and pressure. the main factors that cause phase changes are changes in temperature and pressure. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture. Why Does Pressure Affect Phase Change.
From circuitgonelladrianxm.z22.web.core.windows.net
How To Interpret A Phase Change Diagram Why Does Pressure Affect Phase Change note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. In the picture above, we have a container. phase changes among the various phases of matter depend on temperature and pressure. the main factors that cause phase changes are changes in temperature and pressure. The existence. Why Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Why Does Pressure Affect Phase Change Pressure can also be used to change the phase of the substance. In the picture above, we have a container. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. At the phase transition, such as the. These changes occur when sufficient energy is. the main factors. Why Does Pressure Affect Phase Change.
From wiredatabroriinsisk2b.z22.web.core.windows.net
How To Read A Phase Change Diagram Why Does Pressure Affect Phase Change At the phase transition, such as the. In the picture above, we have a container. describe the relationship between heat (energy), bonding forces, and phase changes. the main factors that cause phase changes are changes in temperature and pressure. The existence of the three phases with. note that, at a fixed temperature, you can change the phase. Why Does Pressure Affect Phase Change.
From shaunmwilliams.com
Chapter 11 Presentation Why Does Pressure Affect Phase Change describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of the substance. The existence of the three phases with. In the picture above, we have a container. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. At the phase transition,. Why Does Pressure Affect Phase Change.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Resource Book Why Does Pressure Affect Phase Change phase changes among the various phases of matter depend on temperature and pressure. These changes occur when sufficient energy is. A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change. Why Does Pressure Affect Phase Change.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Why Does Pressure Affect Phase Change A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a container. At the phase transition, such as the. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing the pressure. the main factors that cause. Why Does Pressure Affect Phase Change.