Bromine Chlorine Molecule . the group 7 elements are also known as the halogens. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. bromine (i) chloride is a chloride of bromine. The three common group 7 elements are chlorine, bromine and iodine.
from www.vectorstock.com
It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group 7 elements are also known as the halogens. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. bromine (i) chloride is a chloride of bromine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen.
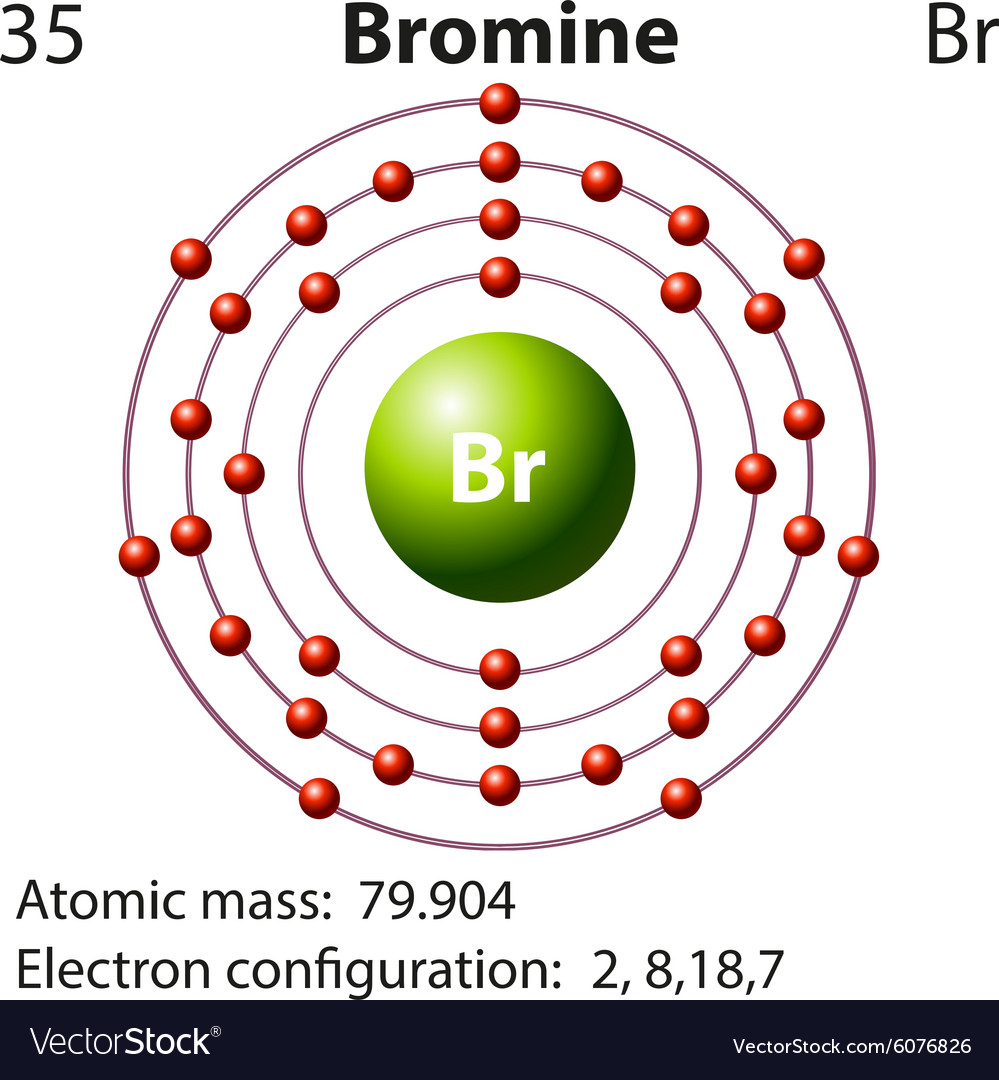
Symbol and electron diagram for bromine Royalty Free Vector
Bromine Chlorine Molecule According to hammond’s postulate, we could say. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. bromine (i) chloride is a chloride of bromine. According to hammond’s postulate, we could say. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens.
From www.youtube.com
Exercise 15.24 Using Mass Spectrometry to Determine if Bromine or Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. bromine (i) chloride is a chloride of bromine. the group. Bromine Chlorine Molecule.
From www.sciencephoto.com
Chlorine molecules, illustration Stock Image C055/3835 Science Bromine Chlorine Molecule the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. this page explains how the m+2 peak in a mass spectrum arises from. Bromine Chlorine Molecule.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Bromine Chlorine Molecule the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. this page explains how the m+2 peak in a mass spectrum arises from the presence of. Bromine Chlorine Molecule.
From fphoto.photoshelter.com
science chemistry halide displacement reaction chlorine postassium Bromine Chlorine Molecule According to hammond’s postulate, we could say. the group 7 elements are also known as the halogens. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are. Bromine Chlorine Molecule.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Chlorine Molecule the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. . Bromine Chlorine Molecule.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Bromine Chlorine Molecule the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. bromine (i) chloride is a chloride of bromine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. the group 7. Bromine Chlorine Molecule.
From fphoto.photoshelter.com
science chemistry halide displacement reaction chlorine postassium Bromine Chlorine Molecule the group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. According to hammond’s postulate, we could say. bromine (i) chloride is a chloride of bromine. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as. Bromine Chlorine Molecule.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine Chlorine Molecule in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. bromine (i) chloride is a chloride of bromine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. . Bromine Chlorine Molecule.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Bromine Chlorine Molecule the group 7 elements are also known as the halogens. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the. Bromine Chlorine Molecule.
From www.slideserve.com
PPT Chem805 Identification of organic and compounds by Bromine Chlorine Molecule the group 7 elements are also known as the halogens. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the group 7 elements are also known as the halogens.. Bromine Chlorine Molecule.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine Chlorine Molecule According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. this page explains how the. Bromine Chlorine Molecule.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine Chlorine Molecule in chlorination, the reaction is exothermic, and the transition state resembles the reactants. According to hammond’s postulate, we could say. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. The three common group 7 elements are chlorine, bromine and iodine. the group 7. Bromine Chlorine Molecule.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Chlorine Molecule It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. bromine (i) chloride is a chloride of bromine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen. Bromine Chlorine Molecule.
From www.vecteezy.com
Collection of molecular chemical models combinations from hydrogen Bromine Chlorine Molecule According to hammond’s postulate, we could say. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group. Bromine Chlorine Molecule.
From www.youtube.com
Chlorine Water + Sodium Bromide YouTube Bromine Chlorine Molecule According to hammond’s postulate, we could say. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. this page explains how the. Bromine Chlorine Molecule.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an. Bromine Chlorine Molecule.
From depositphotos.com
Cl2 chlorine molecule — Stock Vector © MariaShmitt 96911050 Bromine Chlorine Molecule According to hammond’s postulate, we could say. The three common group 7 elements are chlorine, bromine and iodine. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. the group 7 elements are also known as the halogens. The three common group 7 elements are. Bromine Chlorine Molecule.
From dokumen.tips
(PDF) Raman spectra of molecular crystals I. Chlorine, bromine, and Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. According to hammond’s postulate, we could say. this page. Bromine Chlorine Molecule.
From www.numerade.com
SOLVED Write the chemical formula for this molecule carbon hydrogen Bromine Chlorine Molecule the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the seven diatomic elements are. Bromine Chlorine Molecule.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Chlorine Molecule According to hammond’s postulate, we could say. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. this. Bromine Chlorine Molecule.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Bromine Chlorine Molecule in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. the group 7 elements are also known as the halogens. this page explains how the m+2 peak in a. Bromine Chlorine Molecule.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine Chlorine Molecule the group 7 elements are also known as the halogens. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. the group 7 elements are also known as. Bromine Chlorine Molecule.
From sciencechemistri.blogspot.com
Science Chemistry Properties of Chlorine, Bromine, Iodine and Astatine Bromine Chlorine Molecule this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. the group 7 elements are also known as the halogens. the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. The three common group 7 elements are. Bromine Chlorine Molecule.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine Chlorine Molecule in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. The three common group 7 elements. Bromine Chlorine Molecule.
From society6.com
Chlorine Molecule Art Print by RosalisArt Society6 Bromine Chlorine Molecule According to hammond’s postulate, we could say. the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. this page explains how the m+2 peak in a mass spectrum arises from the presence. Bromine Chlorine Molecule.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrCl Bromine monochloride Bromine Chlorine Molecule bromine (i) chloride is a chloride of bromine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the seven diatomic. Bromine Chlorine Molecule.
From www.sciencephoto.com
Chlorine molecules Stock Image A602/0069 Science Photo Library Bromine Chlorine Molecule this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic. bromine (i) chloride is a chloride of bromine. According to hammond’s postulate, we could say. The three common group 7 elements are chlorine, bromine and iodine. in chlorination, the reaction is exothermic, and the. Bromine Chlorine Molecule.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group 7 elements are also known as the halogens. this page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an. Bromine Chlorine Molecule.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Chlorine Molecule the group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. bromine (i) chloride is a. Bromine Chlorine Molecule.
From www.semanticscholar.org
[PDF] Mixed bromine/chlorine transformation products of Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. According to hammond’s postulate, we could say. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. the group. Bromine Chlorine Molecule.
From pediaa.com
Difference Between Bromine and Chlorine Bromine Chlorine Molecule It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. According to hammond’s postulate, we could say. this page explains how the m+2 peak in a mass spectrum arises. Bromine Chlorine Molecule.
From sciencestruck.com
Bromine Vs. Chlorine Bromine Chlorine Molecule The three common group 7 elements are chlorine, bromine and iodine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. bromine (i) chloride is a chloride of bromine. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. It. Bromine Chlorine Molecule.
From pediaa.com
Difference Between Bromine and Chlorine Bromine Chlorine Molecule According to hammond’s postulate, we could say. The three common group 7 elements are chlorine, bromine and iodine. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. bromine (i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial. the group 7. Bromine Chlorine Molecule.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Bromine Chlorine Molecule According to hammond’s postulate, we could say. the seven diatomic elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. this page explains how the m+2 peak in a mass spectrum arises from the presence of. Bromine Chlorine Molecule.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Bromine Chlorine Molecule the group 7 elements are also known as the halogens. According to hammond’s postulate, we could say. in chlorination, the reaction is exothermic, and the transition state resembles the reactants. bromine (i) chloride is a chloride of bromine. The three common group 7 elements are chlorine, bromine and iodine. the seven diatomic elements are iodine, bromine,. Bromine Chlorine Molecule.