Standard Enthalpy Of Formation Decomposition . The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. Each element must be in the. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.
from studylib.net
\[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Each element must be in the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure.
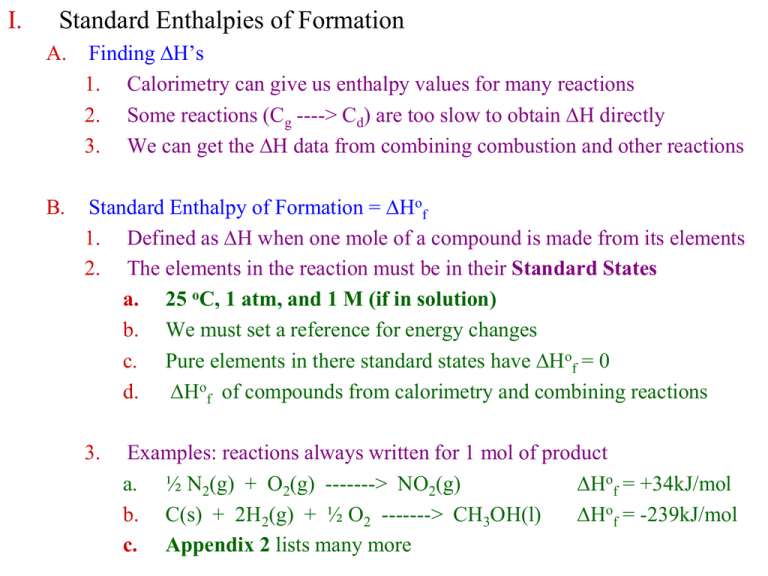
I. Standard Enthalpies of Formation
Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Each element must be in the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and.
From slideplayer.com
Changes in Enthalpy During Chemical Reactions ppt download Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. A standard enthalpy of. Standard Enthalpy Of Formation Decomposition.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Standard Enthalpy Of Formation Decomposition Each element must be in the. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 193 rows the standard enthalpy change of any reaction can be calculated. Standard Enthalpy Of Formation Decomposition.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound. Standard Enthalpy Of Formation Decomposition.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Decomposition Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, also known as the heat of. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. 193 rows the standard enthalpy change of any reaction can be calculated. Standard Enthalpy Of Formation Decomposition.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Decomposition Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. Each element must be in the. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. \[ 6c\left (s, graphite \right ) + 6h_{2}\left. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Each element must be in the. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements. Standard Enthalpy Of Formation Decomposition.
From www.scribd.com
Standard Enthalpy of Formation PDF Solvation Chemical Process Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. A standard enthalpy of formation \. Standard Enthalpy Of Formation Decomposition.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is. Standard Enthalpy Of Formation Decomposition.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Decomposition Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in. Standard Enthalpy Of Formation Decomposition.
From www.gauthmath.com
Solved What does H f stand for? Standard Enthalpy of Formation Standard Enthalpy Of Formation Decomposition 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Each element must be in the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED Calculate the heat of for this process at Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. The standard. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED 1)Baking soda (NaHCO3) on heating as follows 2 Standard Enthalpy Of Formation Decomposition Each element must be in the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is. Standard Enthalpy Of Formation Decomposition.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Enthalpy Of Formation Decomposition \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of. Standard Enthalpy Of Formation Decomposition.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy. Standard Enthalpy Of Formation Decomposition.
From www.toppr.com
53. The standard enthalpies of formation of and H,Og, are 187.8K J Standard Enthalpy Of Formation Decomposition \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Each element must be in the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a. Standard Enthalpy Of Formation Decomposition.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Decomposition.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; The standard enthalpy of formation, also known as the heat of formation, is. Standard Enthalpy Of Formation Decomposition.
From www.chegg.com
Solved The standard enthalpy of of Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Of Formation Decomposition.
From studylib.net
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right. Standard Enthalpy Of Formation Decomposition.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Each element must be in the. A standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Decomposition 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of glucose from the elements at 25°c is. Standard Enthalpy Of Formation Decomposition.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Each element must be in the. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED 4. Use standard enthalpies of formation to calculate the Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Each element must be in the. 193 rows the standard enthalpy change of any reaction can be calculated from. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows the standard enthalpy change. Standard Enthalpy Of Formation Decomposition.
From www.numerade.com
SOLVED Calculate the enthalpy change for the reaction in Standard Enthalpy Of Formation Decomposition Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. 193 rows the standard enthalpy change of any reaction can be calculated from the standard. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
5.7 Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Decomposition Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Decomposition.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Standard Enthalpy Of Formation Decomposition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Each element must be in the. Standard enthalpy of formation is defined as the enthalpy change. Standard Enthalpy Of Formation Decomposition.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Decomposition 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Enthalpy Of Formation Decomposition.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. \[ 6c\left (s, graphite \right ) + 6h_{2}\left (g \right ) + 3o_{2}\left (g \right ) \rightarrow c_{6}h_{12}o_{6}\left (s \right )\; Standard enthalpy of formation is defined as the enthalpy change when one mole of a. Standard Enthalpy Of Formation Decomposition.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Decomposition Each element must be in the. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. 193 rows the standard enthalpy change. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Decomposition Each element must be in the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable. The standard enthalpy of formation. Standard Enthalpy Of Formation Decomposition.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Decomposition 193 rows the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and. Each element must be in the. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Decomposition.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Decomposition The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is the enthalpy change when 1 mol of. Standard Enthalpy Of Formation Decomposition.