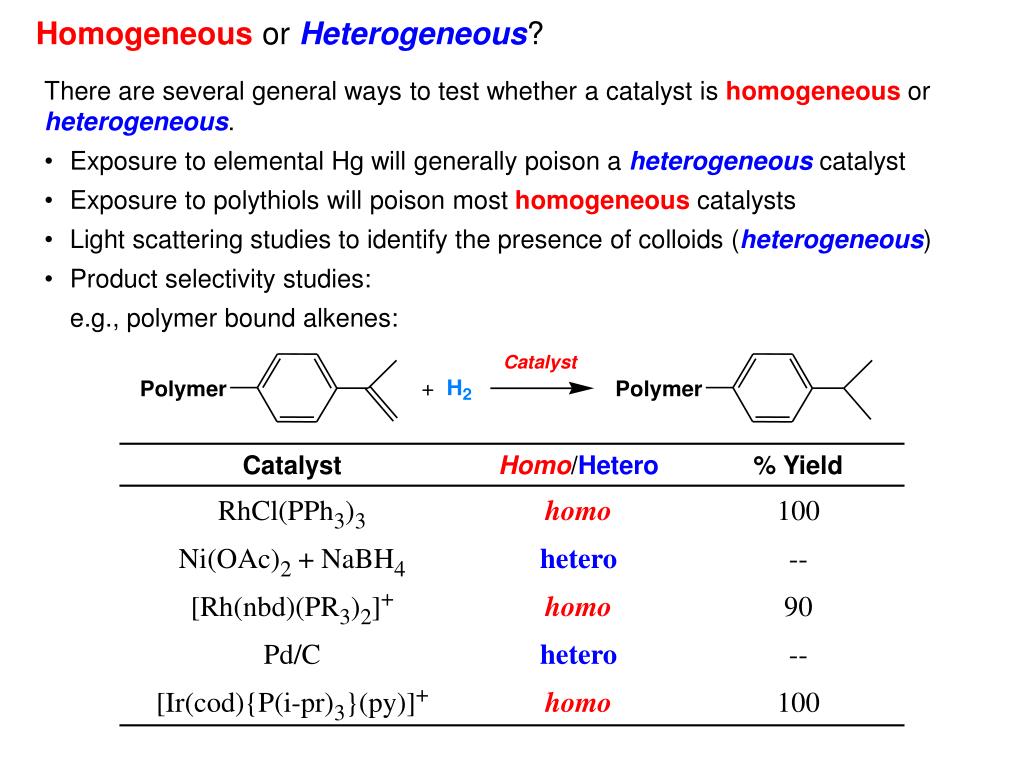
Homogeneous Catalysts With Examples . Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, the catalyst is in aqueous solution, and the reactants are in the same. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is a catalyst which is in the same phase as the reactant.
from www.slideserve.com
This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. For example, the catalyst is in aqueous solution, and the reactants are in the same. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to.
PPT Homogeneous Catalysis Introduction PowerPoint Presentation
Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. For example, the catalyst is in aqueous solution, and the reactants are in the same. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. A homogeneous catalyst is present in the same phase as the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. A homogeneous catalyst is present in the same phase as the reactants. For example, the catalyst is in aqueous solution, and the reactants are in the same. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Homogeneous Catalysts With Examples For example, the catalyst is in aqueous solution, and the reactants are in the same. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. For example, iron (fe) is often used as. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3996834 Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A homogeneous catalyst is present in the same phase as the reactants. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. A homogeneous. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Reaction Rate PowerPoint Presentation, free download ID3534068 Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. A homogeneous catalyst is present in the same phase as the reactants. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. For example, iron (fe) is often used as a catalyst due. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT CHBE 452 Lecture 27 PowerPoint Presentation, free download ID Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, iron (fe) is often used as a catalyst due to its ability. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Homogeneous catalysis PowerPoint Presentation, free download ID Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Homogeneous Catalysts With Examples.
From www.youtube.com
Homogeneous Catalysis Reaction with Mechanisms Physical Chemistry Homogeneous Catalysts With Examples Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, the catalyst is in aqueous solution, and the reactants are in the same. A. Homogeneous Catalysts With Examples.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Homogeneous Catalysts With Examples A homogeneous catalyst is present in the same phase as the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising. Homogeneous Catalysts With Examples.
From www.researchgate.net
Different types of transition metal catalysts for CO 2 RR (a Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. For example, the catalyst is in aqueous solution, and. Homogeneous Catalysts With Examples.
From www.researchgate.net
Examples of homogeneous acid catalysts used in biodiesel production Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. For example, the catalyst is in aqueous. Homogeneous Catalysts With Examples.
From www.youtube.com
Homogeneous Hydrogenation Crabtree's Catalyst Stereoselectivity of Homogeneous Catalysts With Examples A homogeneous catalyst is present in the same phase as the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, the catalyst is in aqueous solution, and the reactants are in the same. This page looks at the the different types of. Homogeneous Catalysts With Examples.
From phys.org
Singleatom catalyst based on homogeneous catalysis prototype for CO2 Homogeneous Catalysts With Examples Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. For example, the catalyst is in aqueous solution, and the reactants are in the same. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For. Homogeneous Catalysts With Examples.
From www.slideshare.net
Catalysis Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. For example, the catalyst is in aqueous solution, and. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Homogeneous Catalysts With Examples For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. There are an extended variety of homogeneous catalysts, ranging. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Organometallic Catalysts PowerPoint Presentation ID997338 Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii). Homogeneous Catalysts With Examples.
From www.scribd.com
Homogeneous Catalysts. Types, Reactions and Applications Catalysis Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. For example, the catalyst is in aqueous solution, and the reactants are in the same. For example, iron (fe) is often used as. Homogeneous Catalysts With Examples.
From www.slideshare.net
Homogeneous catalysis [ MPHARM, MSC, BPHARM, BSC] Homogeneous Catalysts With Examples A homogeneous catalyst is present in the same phase as the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. A homogeneous. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. A homogeneous catalyst is present in the same phase as the reactants. For example, iron (fe) is often used as a catalyst due. Homogeneous Catalysts With Examples.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Homogeneous Catalysts With Examples For example, the catalyst is in aqueous solution, and the reactants are in the same. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii). Homogeneous Catalysts With Examples.
From www.youtube.com
Which one of the following is an example of homogeneous catalysis Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. A homogeneous catalyst is present in the same phase as the reactants. For example, the catalyst is in aqueous solution, and the reactants. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved. Homogeneous Catalysts With Examples.
From www.researchgate.net
Broad classification of the main types of homogeneous and heterogeneous Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. Examples of homogeneous catalysts for arene hydrogenation are rare though it is routinely achieved using catalysts like rh/c under the heterogeneous conditions. For example, iron (fe) is often used as a catalyst due to its ability to form. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Homogeneous Catalysts With Examples.
From www.youtube.com
Types Of Homogeneous Catalysis YouTube Homogeneous Catalysts With Examples A homogeneous catalyst is present in the same phase as the reactants. For example, the catalyst is in aqueous solution, and the reactants are in the same. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A homogeneous catalyst is a catalyst which is in. Homogeneous Catalysts With Examples.
From www.chemistrystudent.com
Homogeneous Catalysis (ALevel) ChemistryStudent Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, the catalyst is in aqueous solution, and the reactants are in the same. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. For example, iron (fe) is often. Homogeneous Catalysts With Examples.
From giokbgwca.blob.core.windows.net
Two Examples Of Catalyst at Stringfellow blog Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. A homogeneous catalyst is present in the same phase as the reactants. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. Examples of homogeneous catalysts. Homogeneous Catalysts With Examples.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Homogeneous Catalysts With Examples For example, the catalyst is in aqueous solution, and the reactants are in the same. A homogeneous catalyst is a catalyst which is in the same phase as the reactant. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, iron (fe) is often. Homogeneous Catalysts With Examples.
From www.youtube.com
What is a Homogeneous Catalyst? YouTube Homogeneous Catalysts With Examples A homogeneous catalyst is a catalyst which is in the same phase as the reactant. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. A homogeneous catalyst is present in the same phase as the reactants. Examples of homogeneous catalysts. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Homogeneous Catalysts With Examples There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. A homogeneous catalyst is a catalyst which is in. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. A homogeneous catalyst is present in the same phase as the reactants. For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Homogeneous Catalysts With Examples For example, iron (fe) is often used as a catalyst due to its ability to form fe (ii) and fe (iii) ions, acting as an oxidising agent and a. A homogeneous catalyst is present in the same phase as the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and. Homogeneous Catalysts With Examples.
From www.researchgate.net
Comparison between heterogeneous, homogeneous, and nanocatalyst Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. For example, iron (fe) is often used as a catalyst due to its ability. Homogeneous Catalysts With Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Homogeneous Catalysts With Examples This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. There are an extended variety of homogeneous catalysts, ranging from brønsted and lewis acids, which are widely used in organic synthesis, to. For example, the catalyst is in aqueous solution, and the reactants are in the same. For. Homogeneous Catalysts With Examples.