Zinc Sulfate And Sodium Hydroxide Precipitate . learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. in effect zinc is not displacing sodium. See examples, tests, and applications of. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. The metal is more reactive. Znso4 + naoh = zn(oh)2 + na2so4 is a double. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. We expect a metal to do this from a hydroxide base if. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. For instance, barium sulfate indicates sulfate. explain that white precipitates can indicate different ions depending on the test. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions.
from projectopenletter.com
learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. The metal is more reactive. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. For instance, barium sulfate indicates sulfate. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. explain that white precipitates can indicate different ions depending on the test. See examples, tests, and applications of. We expect a metal to do this from a hydroxide base if. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate.
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form
Zinc Sulfate And Sodium Hydroxide Precipitate learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. explain that white precipitates can indicate different ions depending on the test. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. See examples, tests, and applications of. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. For instance, barium sulfate indicates sulfate. The metal is more reactive. in effect zinc is not displacing sodium. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. We expect a metal to do this from a hydroxide base if. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Zinc Sulfate And Sodium Hydroxide Precipitate See examples, tests, and applications of. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. Znso4 + naoh = zn(oh)2 + na2so4 is a double. We expect a metal to do this from a hydroxide base if. The metal is more reactive. learn how precipitation reactions occur when insoluble. Zinc Sulfate And Sodium Hydroxide Precipitate.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Sulfate And Sodium Hydroxide Precipitate 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. For instance, barium sulfate indicates sulfate. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. The metal. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Zinc Sulfate And Sodium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. For instance, barium sulfate indicates sulfate. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED16. When solutions of aluminum sulfate and sodium hydroxide are Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. We expect a metal to do this from a hydroxide base if. Znso4 + naoh = zn(oh)2 + na2so4 is. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.dreamstime.com
Zinc Powder, CopperII Sulfate and Sodium Hydroxide Pellets in Test Tube Zinc Sulfate And Sodium Hydroxide Precipitate For instance, barium sulfate indicates sulfate. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. See examples, tests, and applications of. in effect zinc is not displacing sodium. explain that white precipitates can indicate different ions depending on the test. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to predict. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
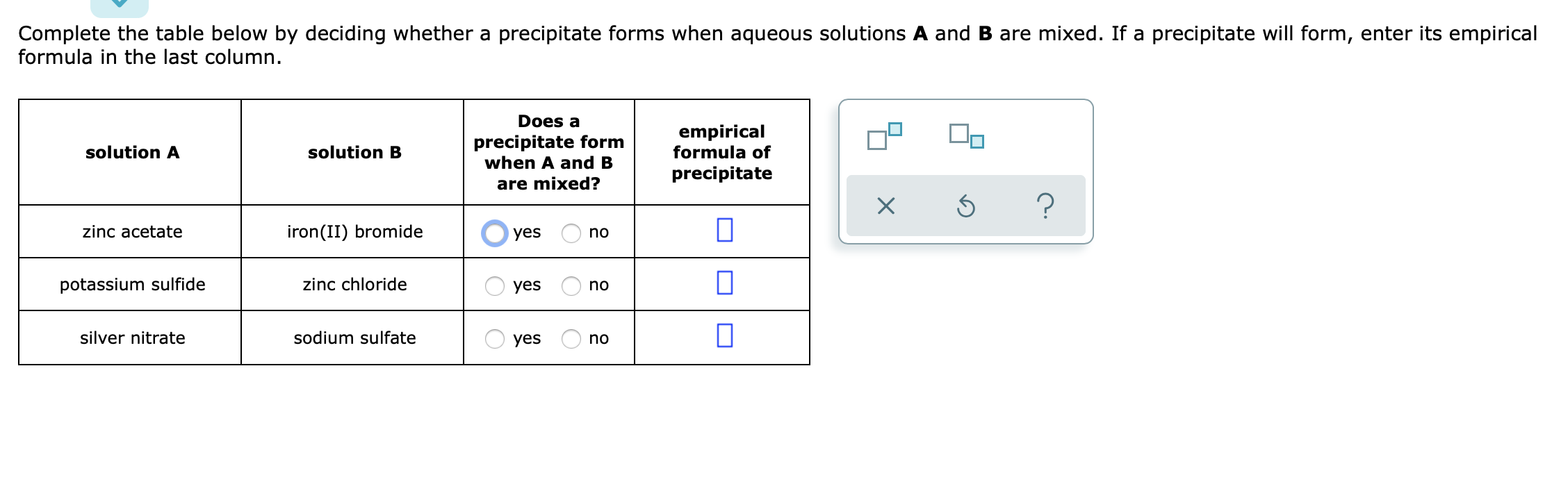
SOLVED Complete the table below by deciding whether precipitate forms Zinc Sulfate And Sodium Hydroxide Precipitate zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. The metal is more reactive. learn how to predict and identify precipitation. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.researchgate.net
Comparison of Zn content in the precipitate after precipitation with Zinc Sulfate And Sodium Hydroxide Precipitate For instance, barium sulfate indicates sulfate. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. learn how to predict and identify. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.essentialchemicalindustry.org
Zinc Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. See examples, tests, and applications of. The metal is more reactive. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. We expect a metal to do this from a. Zinc Sulfate And Sodium Hydroxide Precipitate.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Sulfate And Sodium Hydroxide Precipitate explain that white precipitates can indicate different ions depending on the test. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. See examples, tests, and applications of. For instance, barium sulfate indicates sulfate. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. The metal is more reactive. Znso4. Zinc Sulfate And Sodium Hydroxide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. in effect zinc is not displacing sodium. For instance, barium sulfate indicates sulfate. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. learn how to identify, predict. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.teachoo.com
What do you mean by a precipitation reaction? Explain by examples Zinc Sulfate And Sodium Hydroxide Precipitate See examples, tests, and applications of. The metal is more reactive. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. explain that white precipitates can indicate different ions depending on the test. 7 rows learn how. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.semanticscholar.org
Figure 1 from Effect of pH, concentration and temperature on copper and Zinc Sulfate And Sodium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. explain that white precipitates can indicate different ions depending on the test. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. See examples, tests, and applications of. The metal is more reactive. . Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.nagwa.com
Question Video Recalling the Color of the Precipitate Forms When Zinc Sulfate And Sodium Hydroxide Precipitate learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. in effect. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.researchgate.net
a Color change and formation of white precipitate, b mechanism of Zinc Sulfate And Sodium Hydroxide Precipitate zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. For instance, barium sulfate indicates sulfate. We expect a metal to do this from a hydroxide base if. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. 7 rows learn how to. Zinc Sulfate And Sodium Hydroxide Precipitate.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. See examples, tests, and applications of. We expect a metal to do this from a hydroxide base if. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. For. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Sulfate And Sodium Hydroxide Precipitate learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. Znso4 + naoh = zn(oh)2 + na2so4 is a double. zinc sulfate + sodium hydroxide = zinc hydroxide +. Zinc Sulfate And Sodium Hydroxide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Sulfate And Sodium Hydroxide Precipitate learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. See examples, tests, and applications of. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. explain that. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED Nickel(II) sulfate solution reacts with sodium hydroxide Zinc Sulfate And Sodium Hydroxide Precipitate The metal is more reactive. See examples, tests, and applications of. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. in effect zinc is not displacing sodium. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. 7 rows learn how to test for metal ions using sodium hydroxide. Zinc Sulfate And Sodium Hydroxide Precipitate.
From fineartamerica.com
Zinc Hydroxide Precipitate Photograph by Andrew Lambert Photography Zinc Sulfate And Sodium Hydroxide Precipitate See examples, tests, and applications of. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. explain that white precipitates can indicate different ions depending on the test. The. Zinc Sulfate And Sodium Hydroxide Precipitate.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Zinc Sulfate And Sodium Hydroxide Precipitate learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. learn how precipitation reactions occur when. Zinc Sulfate And Sodium Hydroxide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. We expect a metal to do this from a hydroxide base if. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to identify, predict and apply precipitation reactions, a subclass of exchange. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED Does precipitate form when A and B are mixed? empirical formula Zinc Sulfate And Sodium Hydroxide Precipitate explain that white precipitates can indicate different ions depending on the test. The metal is more reactive. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. We expect a metal to do this from a hydroxide base if. in effect zinc is not displacing sodium. learn how to identify, predict. Zinc Sulfate And Sodium Hydroxide Precipitate.
From cartoondealer.com
Sodium Hydroxide Pellets, Zinc Granules And Copper(II) Sulfate In Test Zinc Sulfate And Sodium Hydroxide Precipitate See examples, tests, and applications of. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. The metal is more reactive. 7 rows learn how to test for. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.topperlearning.com
What do you observe when sodium hydroxide solution is added to zinc Zinc Sulfate And Sodium Hydroxide Precipitate learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. For instance, barium sulfate indicates sulfate. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. in effect zinc is not displacing sodium. learn. Zinc Sulfate And Sodium Hydroxide Precipitate.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Sulfate And Sodium Hydroxide Precipitate learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. We expect a metal to do this from a hydroxide base if. in effect zinc is not displacing sodium. For instance, barium sulfate indicates sulfate. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. 7 rows learn how. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.alamy.com
Zinc hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Zinc Sulfate And Sodium Hydroxide Precipitate zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. explain that white precipitates can indicate different ions depending on the test. See examples, tests, and applications of. in effect zinc is not displacing sodium. learn how to use. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Zinc Sulfate And Sodium Hydroxide Precipitate 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. See examples, tests, and applications of. learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. in effect zinc is not displacing sodium. The metal is more reactive. . Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.youtube.com
Reaction of zinc sulphate (ZnSO4) with Ammonium hydroxide (NH4OH) YouTube Zinc Sulfate And Sodium Hydroxide Precipitate learn how to use the solubility rules for inorganic compounds to determine if a precipitate will form when two aqueous solutions are mixed. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. We expect a metal to do this from a. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED Texts Complete the table below by deciding whether a Zinc Sulfate And Sodium Hydroxide Precipitate learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. zinc sulfate + sodium hydroxide = zinc hydroxide + sodium sulfate. The metal is. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.dreamstime.com
Sodium Hydroxide Pellets, Zinc Granules and Copper(II) Sulfate in Test Zinc Sulfate And Sodium Hydroxide Precipitate See examples, tests, and applications of. explain that white precipitates can indicate different ions depending on the test. We expect a metal to do this from a hydroxide base if. For instance, barium sulfate indicates sulfate. The metal is more reactive. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to predict and identify precipitation. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.bartleby.com
Answered Complete the table below by deciding… bartleby Zinc Sulfate And Sodium Hydroxide Precipitate Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. The metal is more reactive. explain that white precipitates can indicate different ions depending on the test. learn how to use the solubility rules for inorganic compounds to determine. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Sulfate And Sodium Hydroxide Precipitate explain that white precipitates can indicate different ions depending on the test. Znso4 + naoh = zn(oh)2 + na2so4 is a double. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. We expect a metal to do this from a hydroxide base if. learn how to identify, predict and apply precipitation. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED Does a solution A solution B precipitate form when A and B are Zinc Sulfate And Sodium Hydroxide Precipitate explain that white precipitates can indicate different ions depending on the test. learn how to identify, predict and apply precipitation reactions, a subclass of exchange reactions that yield insoluble products. in effect zinc is not displacing sodium. Znso4 + naoh = zn(oh)2 + na2so4 is a double. We expect a metal to do this from a hydroxide. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Zinc Sulfate And Sodium Hydroxide Precipitate explain that white precipitates can indicate different ions depending on the test. learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. The metal is more reactive. Znso4 + naoh = zn(oh)2 + na2so4 is a double. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions.. Zinc Sulfate And Sodium Hydroxide Precipitate.
From www.dreamstime.com
Interaction of Solutions of Sodium Hydroxide and Copper II Sulfate. the Zinc Sulfate And Sodium Hydroxide Precipitate learn how precipitation reactions occur when insoluble salts form from soluble ions in aqueous solutions. See examples, tests, and applications of. learn how to predict and identify precipitation reactions, a type of double displacement reaction, using solubility. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. learn how to use. Zinc Sulfate And Sodium Hydroxide Precipitate.