Acid Base Titration Lab Experiment . Titration is an analytical quantitative technique used to determine the concentration of a solute; Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Eye protection must be worn. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. They can also investigate the effect of co 2 dissolving on the ph of water. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with an unknown molarity. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and.
from www.chegg.com
The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The analyte (titrand) is the solution with an unknown molarity. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; Eye protection must be worn. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. They can also investigate the effect of co 2 dissolving on the ph of water. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct.
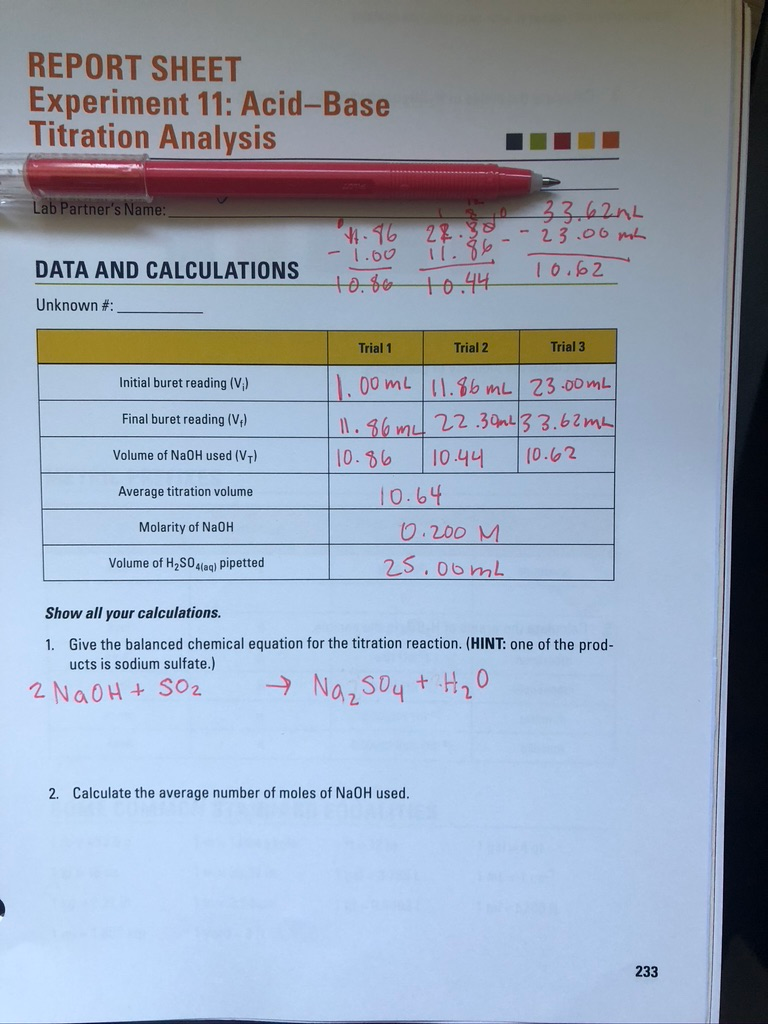
Solved REPORT SHEET Experiment 11 AcidBase Titration
Acid Base Titration Lab Experiment Eye protection must be worn. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. They can also investigate the effect of co 2 dissolving on the ph of water. The analyte (titrand) is the solution with an unknown molarity. Eye protection must be worn. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. The reagent (titrant) is the solution with a known molarity that will react with the analyte. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Acid Base Titration Lab Experiment The analyte (titrand) is the solution with an unknown molarity. Titration is an analytical quantitative technique used to determine the concentration of a solute; Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Be able to determine the k a or k b from ph data associated with the titration of. Acid Base Titration Lab Experiment.
From mungfali.com
Acid Base Titration Experiment Acid Base Titration Lab Experiment Titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. They can also investigate the effect of co 2 dissolving on the ph of water. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. As an extension,. Acid Base Titration Lab Experiment.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Acid Base Titration Lab Experiment Eye protection must be worn. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is an analytical quantitative technique used to determine the concentration of a solute; The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. They can also. Acid Base Titration Lab Experiment.
From stock.adobe.com
Laboratory experiment of acid base titration with glass burette and Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide,. Acid Base Titration Lab Experiment.
From mungfali.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. They can also investigate the effect of co 2 dissolving on the ph of water. The analyte (titrand) is the solution with an unknown molarity. Eye protection must be worn. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Acid Base Titration Lab Experiment.
From www.youtube.com
Experiment for the Determination of amount of citric acid in lemon Acid Base Titration Lab Experiment Eye protection must be worn. They can also investigate the effect of co 2 dissolving on the ph of water. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Titration is an analytical quantitative technique used to determine the concentration of a solute; In this experiment students neutralise sodium hydroxide with. Acid Base Titration Lab Experiment.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Acid Base Titration Lab Experiment They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Eye protection must be worn. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; They can also investigate. Acid Base Titration Lab Experiment.
From www.scribd.com
Acid Base Titration Experiment 4 PDF Acid Base Titration Lab Experiment Titration is an analytical quantitative technique used to determine the concentration of a solute; Eye protection must be worn. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. As an extension,. Acid Base Titration Lab Experiment.
From www.nagwa.com
Question Video Explaining Why the Conical Flask Is Placed on a White Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an unknown molarity. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Acid Base Titration Lab Experiment.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration Lab Experiment The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in. Acid Base Titration Lab Experiment.
From www.flinnsci.com
360 Science Titrations and the Study of AcidBase Chemistry Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. They can also investigate the effect of co 2 dissolving on the ph of water. Titration is an analytical quantitative. Acid Base Titration Lab Experiment.
From mavink.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Experiment In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Eye protection must be worn. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Be. Acid Base Titration Lab Experiment.
From fineartamerica.com
Acidbase Titration by Science Source Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. They can also investigate the effect of co 2 dissolving on the ph of water. Titration is an. Acid Base Titration Lab Experiment.
From titration-lab1.weebly.com
Data & Calculations Acid Base Titration Lab Acid Base Titration Lab Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. They can also investigate the effect of co 2 dissolving on the ph of water. Eye protection must be worn. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with an unknown. Acid Base Titration Lab Experiment.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is. Acid Base Titration Lab Experiment.
From www.vernier.com
AcidBase Titration > Experiment 7 from Advanced Chemistry with Vernier Acid Base Titration Lab Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. The purpose of this experiment is to observe the titration of. Acid Base Titration Lab Experiment.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Lab Experiment The analyte (titrand) is the solution with an unknown molarity. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid. Acid Base Titration Lab Experiment.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. They can also investigate the effect of co 2 dissolving on the ph of water. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Eye protection must be worn. Be able to. Acid Base Titration Lab Experiment.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Acid Base Titration Lab Experiment They can also investigate the effect of co 2 dissolving on the ph of water. Titration is an analytical quantitative technique used to determine the concentration of a solute; The reagent (titrant) is the solution with a known molarity that will react with the analyte. As an extension, ask learners to titrate the original hcl against the naoh to check. Acid Base Titration Lab Experiment.
From studylib.net
Acid/Base Titration Lab Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. The analyte (titrand) is the solution with an unknown molarity. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Eye protection must be worn. The reagent (titrant) is the solution with a. Acid Base Titration Lab Experiment.
From www.dreamstime.com
The Chemist Adds Solutions with Precision, Ensuring Accurate Acid Base Titration Lab Experiment Titration is an analytical quantitative technique used to determine the concentration of a solute; As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Read our standard health and safety guidance and. Acid Base Titration Lab Experiment.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Experiment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The purpose of this experiment is to observe the titration of hydrochloric. Acid Base Titration Lab Experiment.
From mavink.com
Acid Base Titration Simulation Acid Base Titration Lab Experiment The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Titration is an analytical quantitative technique used to determine the concentration of a solute; As an extension, ask learners. Acid Base Titration Lab Experiment.
From mungfali.com
Acid Base Titration Method Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Eye protection must be worn. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. As an. Acid Base Titration Lab Experiment.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Eye protection must be worn. Titration is an analytical quantitative technique used to determine the concentration of a solute; They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with. Acid Base Titration Lab Experiment.
From www.sliderbase.com
Titration Acid Base Titration Lab Experiment The analyte (titrand) is the solution with an unknown molarity. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. The reagent (titrant) is the solution with a known molarity that will react with the analyte. As an extension, ask learners to titrate the original hcl against the naoh to check the. Acid Base Titration Lab Experiment.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. They can also investigate the effect of co 2 dissolving on the ph of water. In this experiment students neutralise sodium hydroxide with hydrochloric acid to. Acid Base Titration Lab Experiment.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Lab Experiment The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. The reagent (titrant). Acid Base Titration Lab Experiment.
From mungfali.com
Acid Base Titration Procedure Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Eye protection must be worn. Be able to determine the k a or k b from ph data associated with the titration of. Acid Base Titration Lab Experiment.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Lab Experiment As an extension, ask learners to titrate the original hcl against the naoh to check the concentration is correct. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They can also. Acid Base Titration Lab Experiment.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Acid Base Titration Lab Experiment Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Titration is an analytical quantitative technique used to determine the concentration of a solute; Eye protection must be worn. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and.. Acid Base Titration Lab Experiment.
From www.vernier.com
AcidBase Titration > Experiment 24 from Chemistry with Vernier Acid Base Titration Lab Experiment They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The analyte (titrand) is the solution with an unknown molarity. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and. Titration is an analytical quantitative technique used to determine the concentration of. Acid Base Titration Lab Experiment.
From stock.adobe.com
Acid base titration experiment and phases of color change during Acid Base Titration Lab Experiment They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is an analytical quantitative technique used to determine the concentration of a solute; Read our standard health and safety guidance and carry out a risk assessment before running any live practical. Be able to determine the k a or k b from ph data. Acid Base Titration Lab Experiment.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Acid Base Titration Lab Experiment Titration is an analytical quantitative technique used to determine the concentration of a solute; Eye protection must be worn. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Read our standard health and safety guidance and carry out a risk assessment before running any live practical. The reagent (titrant) is the solution with a. Acid Base Titration Lab Experiment.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Acid Base Titration Lab Experiment They can also investigate the effect of co 2 dissolving on the ph of water. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titration is an analytical quantitative technique used to determine the concentration. Acid Base Titration Lab Experiment.