Catalyst Change The Equilibrium Constant . Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Generally, a catalyst will help a. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The only thing that changes an equilibrium constant is a change of temperature. [h 2] and [i 2]. Changes in temperature change the equilibrium constant kc. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. For an endothermic reaction such as: $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. One thing i cannot get my head around is the effect of. The only thing that changes an equilibrium constant is a change of. Effect of change of volume, pressure; The position of equilibrium is not changed if you add (or change) a catalyst. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions.
from www.slideserve.com
If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Effect of change of volume, pressure; Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. For an endothermic reaction such as: Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The position of equilibrium is not changed if you add (or change) a catalyst. [h 2] and [i 2]. The only thing that changes an equilibrium constant is a change of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.
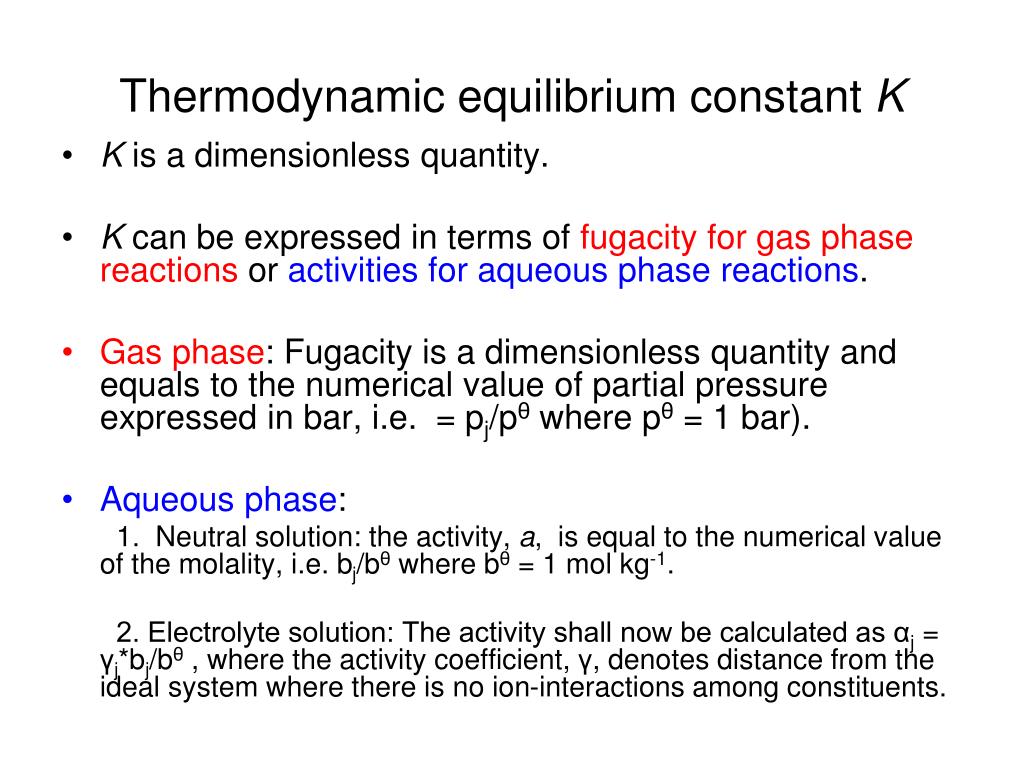
PPT Thermodynamic equilibrium constant K PowerPoint Presentation
Catalyst Change The Equilibrium Constant [h 2] and [i 2]. The only thing that changes an equilibrium constant is a change of. One thing i cannot get my head around is the effect of. Generally, a catalyst will help a. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. [h 2] and [i 2]. Effect of change of volume, pressure; For an endothermic reaction such as: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. The position of equilibrium is not changed if you add (or change) a catalyst. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. The only thing that changes an equilibrium constant is a change of temperature. Changes in temperature change the equilibrium constant kc. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.
From www.researchgate.net
Reactions and equilibrium constant equations. Download Scientific Diagram Catalyst Change The Equilibrium Constant If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Changes in temperature change the equilibrium constant kc. Generally, a catalyst will help a. Equilibrium constants. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Catalyst Change The Equilibrium Constant Generally, a catalyst will help a. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. The only thing that changes an equilibrium constant is a change of. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Changes in temperature change the equilibrium. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Review Expressions of the thermodynamic equilibrium constant K Catalyst Change The Equilibrium Constant For an endothermic reaction such as: Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Generally, a catalyst will help a. One thing i cannot get my head around is the effect of.. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chapter 13 Equilibrium PowerPoint Presentation, free download Catalyst Change The Equilibrium Constant Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. The position of equilibrium is. Catalyst Change The Equilibrium Constant.
From haipernews.com
How To Find Q Equilibrium Constant Haiper Catalyst Change The Equilibrium Constant [h 2] and [i 2]. Changes in temperature change the equilibrium constant kc. For an endothermic reaction such as: The only thing that changes an equilibrium constant is a change of temperature. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Generally, a catalyst will. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Catalyst Change The Equilibrium Constant Changes in temperature change the equilibrium constant kc. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The only thing that changes an equilibrium constant is a change of temperature. Effect of change of volume, pressure; A. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID5546502 Catalyst Change The Equilibrium Constant [h 2] and [i 2]. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. For an endothermic reaction such as: The position of equilibrium is not changed if you add (or change) a catalyst. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. One thing i. Catalyst Change The Equilibrium Constant.
From slideplayer.com
Chapter 13 Chemical Equilibrium ppt download Catalyst Change The Equilibrium Constant If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. One thing i cannot get my head around is the effect of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. For an endothermic reaction such as: Generally, a. Catalyst Change The Equilibrium Constant.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Catalyst Change The Equilibrium Constant Effect of change of volume, pressure; For an endothermic reaction such as: A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. One thing i cannot get my head around is the effect of. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed.. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Catalyst Change The Equilibrium Constant Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Generally, a catalyst will help a. The only thing that changes an equilibrium constant is a change of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. For an endothermic reaction such as:. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Catalyst Change The Equilibrium Constant Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. Changes in temperature change the equilibrium constant kc. One thing i cannot get my head around is the effect of. Le chatelier discovered that equilibrium adjusts the forward. Catalyst Change The Equilibrium Constant.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube Catalyst Change The Equilibrium Constant One thing i cannot get my head around is the effect of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Effect of change of. Catalyst Change The Equilibrium Constant.
From worksheetmediastanley.z21.web.core.windows.net
Equilibrium Constant Kc Formula Catalyst Change The Equilibrium Constant Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Effect of change of volume,. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chemistry Equilibrium PowerPoint Presentation, free download ID Catalyst Change The Equilibrium Constant Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. [h 2] and [i 2]. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. The only thing that changes an equilibrium constant is a change of temperature. Le chatelier discovered that equilibrium adjusts the. Catalyst Change The Equilibrium Constant.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Catalyst Change The Equilibrium Constant The only thing that changes an equilibrium constant is a change of temperature. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. For an endothermic reaction such as: [h 2] and [i 2]. One thing i cannot get my head around is the effect of. A catalyst speeds up both the forward and. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Catalyst Change The Equilibrium Constant For an endothermic reaction such as: One thing i cannot get my head around is the effect of. The only thing that changes an equilibrium constant is a change of. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Equilibrium constants aren't changed if you. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium USING THE EQUILIBRIUM CONSTANT Catalyst Change The Equilibrium Constant The only thing that changes an equilibrium constant is a change of temperature. One thing i cannot get my head around is the effect of. Effect of change of volume, pressure; A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. $\begingroup$ i want to be able to understand shifts. Catalyst Change The Equilibrium Constant.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry Catalyst Change The Equilibrium Constant The only thing that changes an equilibrium constant is a change of temperature. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. One thing i cannot get my head around is the effect. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Unit 3 Equilibrium and pH PowerPoint Presentation, free download Catalyst Change The Equilibrium Constant Changes in temperature change the equilibrium constant kc. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. [h 2] and [i 2]. The position of equilibrium is not changed if you add (or change) a catalyst. Generally, a catalyst will help a. Le chatelier discovered that equilibrium adjusts the forward and backward reactions. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID7054910 Catalyst Change The Equilibrium Constant Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The position of equilibrium is not changed if you add (or change) a catalyst. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Changes in temperature change the equilibrium constant kc. The only thing. Catalyst Change The Equilibrium Constant.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Catalyst Change The Equilibrium Constant One thing i cannot get my head around is the effect of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Changes in temperature change. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium Constant (K eq ) PowerPoint Presentation, free Catalyst Change The Equilibrium Constant Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. The position of equilibrium is not changed if you add (or change) a catalyst. $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. The only thing that changes an. Catalyst Change The Equilibrium Constant.
From www.numerade.com
SOLVED For gas phase reaction, the value of the equilibrium constant Catalyst Change The Equilibrium Constant Generally, a catalyst will help a. Effect of change of volume, pressure; Changes in temperature change the equilibrium constant kc. The position of equilibrium is not changed if you add (or change) a catalyst. For an endothermic reaction such as: If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst.. Catalyst Change The Equilibrium Constant.
From alevelchemistry.co.uk
Equilibrium Constants ALevel Chemistry Revision Notes Catalyst Change The Equilibrium Constant $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. [h 2] and [i 2]. The only thing that changes an equilibrium constant is a change of. Generally, a catalyst will help a. A. Catalyst Change The Equilibrium Constant.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Change The Equilibrium Constant Generally, a catalyst will help a. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The only thing that changes an equilibrium constant is a change of temperature. Changes in temperature change the equilibrium constant kc. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium USING THE EQUILIBRIUM CONSTANT Catalyst Change The Equilibrium Constant For an endothermic reaction such as: A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The only thing. Catalyst Change The Equilibrium Constant.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Catalyst Change The Equilibrium Constant $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. Effect of change of volume, pressure; One thing i cannot get my head around is the effect of. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The only thing that changes an equilibrium constant is a change. Catalyst Change The Equilibrium Constant.
From www.youtube.com
Effect of Catalyst on Equilibrium Constant, Chemistry Lecture Sabaq Catalyst Change The Equilibrium Constant Effect of change of volume, pressure; One thing i cannot get my head around is the effect of. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The position of equilibrium is not changed if you add (or change) a catalyst. The only thing that changes an equilibrium constant is a change of temperature.. Catalyst Change The Equilibrium Constant.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and Catalyst Change The Equilibrium Constant Generally, a catalyst will help a. For an endothermic reaction such as: Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. One thing i cannot get my head around is the effect of. The. Catalyst Change The Equilibrium Constant.
From slideplayer.com
Chemical Equilibria (Gaseous Systems) ppt download Catalyst Change The Equilibrium Constant [h 2] and [i 2]. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Effect of change of volume, pressure; Generally, a catalyst will help. Catalyst Change The Equilibrium Constant.
From classnotes.org.in
Characteristics Of Equilibrium Constant Chemistry, Class 11, Equilibrium Catalyst Change The Equilibrium Constant Changes in temperature change the equilibrium constant kc. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. $\begingroup$ i want to be able to understand. Catalyst Change The Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Catalyst Change The Equilibrium Constant Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. Catalyst Change The Equilibrium Constant.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Change The Equilibrium Constant [h 2] and [i 2]. If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. Le chatelier discovered that equilibrium adjusts the forward and backward reactions in such a way as to accept the changes affecting the equilibrium conditions. One thing i cannot get my head around is the effect. Catalyst Change The Equilibrium Constant.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Catalyst Change The Equilibrium Constant $\begingroup$ i want to be able to understand shifts in equilibrium from the maxwell boltzmann distribution. The position of equilibrium is not changed if you add (or change) a catalyst. For an endothermic reaction such as: If all other conditions stay the same, the equilibrium constant kc is not affected by the presence of a catalyst. The only thing that. Catalyst Change The Equilibrium Constant.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Catalyst Change The Equilibrium Constant Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is not changed if you add (or change) a catalyst. The only thing that changes an equilibrium constant is a change of. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in. Catalyst Change The Equilibrium Constant.