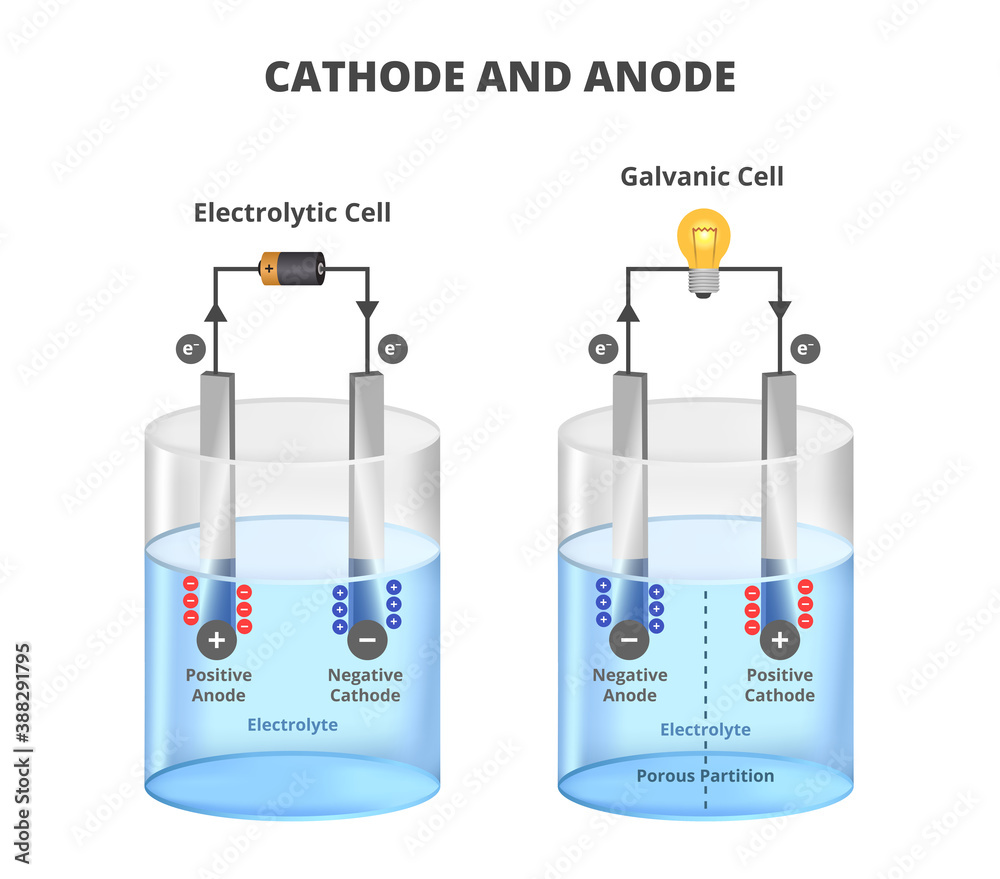
Electrolysis Anode And Cathode Material . The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half.
from stock.adobe.com
In this section, we discuss the recent advances in the innovations of membrane electrolyzers. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,.
Vector scientific illustration of the electrolysis processes. Set of
Electrolysis Anode And Cathode Material The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The electrolysis of aqueous sodium chloride. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The. Electrolysis Anode And Cathode Material.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In this section, we discuss the recent advances in the innovations of membrane electrolyzers.. Electrolysis Anode And Cathode Material.
From www.coursehero.com
Electrolysis Chemistry for Majors Atoms First Course Hero Electrolysis Anode And Cathode Material When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte,. Electrolysis Anode And Cathode Material.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. When. Electrolysis Anode And Cathode Material.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Electrolysis Anode And Cathode Material In this section, we discuss the recent advances in the innovations of membrane electrolyzers. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The electrolysis of aqueous sodium chloride. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The combination of electrocatalytic anodic. Electrolysis Anode And Cathode Material.
From www.thoughtco.com
How to Define Anode and Cathode Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. In this section, we discuss the recent advances in the innovations of membrane electrolyzers.. Electrolysis Anode And Cathode Material.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The electrolysis of aqueous sodium chloride. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In pem water electrolysis, water is electrochemically split into. Electrolysis Anode And Cathode Material.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The. Electrolysis Anode And Cathode Material.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The. Electrolysis Anode And Cathode Material.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In alkaline electrolyzers, the anode. Electrolysis Anode And Cathode Material.
From 2012books.lardbucket.org
Electrolysis Electrolysis Anode And Cathode Material The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize. Electrolysis Anode And Cathode Material.
From forumautomation.com
6 Differences between Anode and Cathode Electronics Industrial Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. When aqueous solutions of ionic compounds. Electrolysis Anode And Cathode Material.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The difference in chemical potentials. Electrolysis Anode And Cathode Material.
From guidewiringstrunting.z14.web.core.windows.net
Cathode Charge In Electrolysis Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The combination of electrocatalytic anodic oxidation with cathodic reduction. Electrolysis Anode And Cathode Material.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The combination of electrocatalytic anodic. Electrolysis Anode And Cathode Material.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Electrolysis Anode And Cathode Material In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective. Electrolysis Anode And Cathode Material.
From circuitlibscombrid.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. When aqueous solutions of ionic compounds. Electrolysis Anode And Cathode Material.
From www.shutterstock.com
Illustration Electrolysis Diagram Cathode Anode Stock Vector (Royalty Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The difference in chemical potentials between the cathode and anode. Electrolysis Anode And Cathode Material.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrolysis Anode And Cathode Material The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In alkaline electrolyzers, the anode and cathode. Electrolysis Anode And Cathode Material.
From instrumentationtools.com
Difference between Anode and Cathode Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The electrolysis of aqueous sodium chloride. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium.. Electrolysis Anode And Cathode Material.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In this section, we discuss the recent advances in the innovations of membrane. Electrolysis Anode And Cathode Material.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrolysis Anode And Cathode Material When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The difference in chemical potentials between the cathode and anode electrolytes. Electrolysis Anode And Cathode Material.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr Electrolysis Anode And Cathode Material The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly. Electrolysis Anode And Cathode Material.
From mavink.com
Anode Cathode Diagram Electrolysis Anode And Cathode Material The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The electrolysis of aqueous sodium chloride. In pem water electrolysis, water. Electrolysis Anode And Cathode Material.
From stock.adobe.com
Electrolysis of Water.electrolysis of acidified water with pt cathode Electrolysis Anode And Cathode Material In this section, we discuss the recent advances in the innovations of membrane electrolyzers. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. When aqueous solutions of ionic compounds are electrolyzed, the. Electrolysis Anode And Cathode Material.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrolysis Anode And Cathode Material In this section, we discuss the recent advances in the innovations of membrane electrolyzers. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The electrolysis of aqueous sodium chloride. The difference in chemical potentials between the cathode and. Electrolysis Anode And Cathode Material.
From www.researchgate.net
Galvanostatic electrolysis using CF anode and Pb anode at cathode Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In this section, we discuss the recent advances in the innovations of membrane. Electrolysis Anode And Cathode Material.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. When aqueous. Electrolysis Anode And Cathode Material.
From userpartfrieda.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrolysis Anode And Cathode Material The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In. Electrolysis Anode And Cathode Material.
From byjus.com
In the electrolytic refining of a metal “M”, what would you take as the Electrolysis Anode And Cathode Material The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. The electrolysis of aqueous sodium chloride. In. Electrolysis Anode And Cathode Material.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Electrolysis Anode And Cathode Material The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The difference in chemical. Electrolysis Anode And Cathode Material.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch Electrolysis Anode And Cathode Material In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. When aqueous solutions of ionic. Electrolysis Anode And Cathode Material.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrolysis Anode And Cathode Material In this section, we discuss the recent advances in the innovations of membrane electrolyzers. The electrolysis of aqueous sodium chloride. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half. The difference in chemical potentials between the cathode and. Electrolysis Anode And Cathode Material.
From rodneyyouthmata.blogspot.com
Anode and Cathode in Electrolysis Electrolysis Anode And Cathode Material The electrolysis of aqueous sodium chloride. In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. In alkaline electrolyzers, the anode and cathode electrodes are immersed in a liquid alkaline electrolyte, most commonly potassium. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the. Electrolysis Anode And Cathode Material.
From www.bajeczneobrazy.pl
Electrolysis chemical technique explanation for DC production outline Electrolysis Anode And Cathode Material In pem water electrolysis, water is electrochemically split into hydrogen and oxygen at their respective electrodes such as hydrogen. The combination of electrocatalytic anodic oxidation with cathodic reduction can not only maximize the return of energy investment,. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In. Electrolysis Anode And Cathode Material.