Explain Solid Liquid Equilibrium With An Example . The curves represent the points. It is a familiar fact that pure substances tend to exist in one of three distinct states: Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this kind of behavior. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912).
from www.mdpi.com
Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this kind of behavior. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). The curves represent the points. It is a familiar fact that pure substances tend to exist in one of three distinct states:
Molecules Free FullText Modeling of SolidLiquid Equilibria in
Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: The curves represent the points. In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are many examples of pairs of compounds that show this kind of behavior. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). It is a familiar fact that pure substances tend to exist in one of three distinct states:
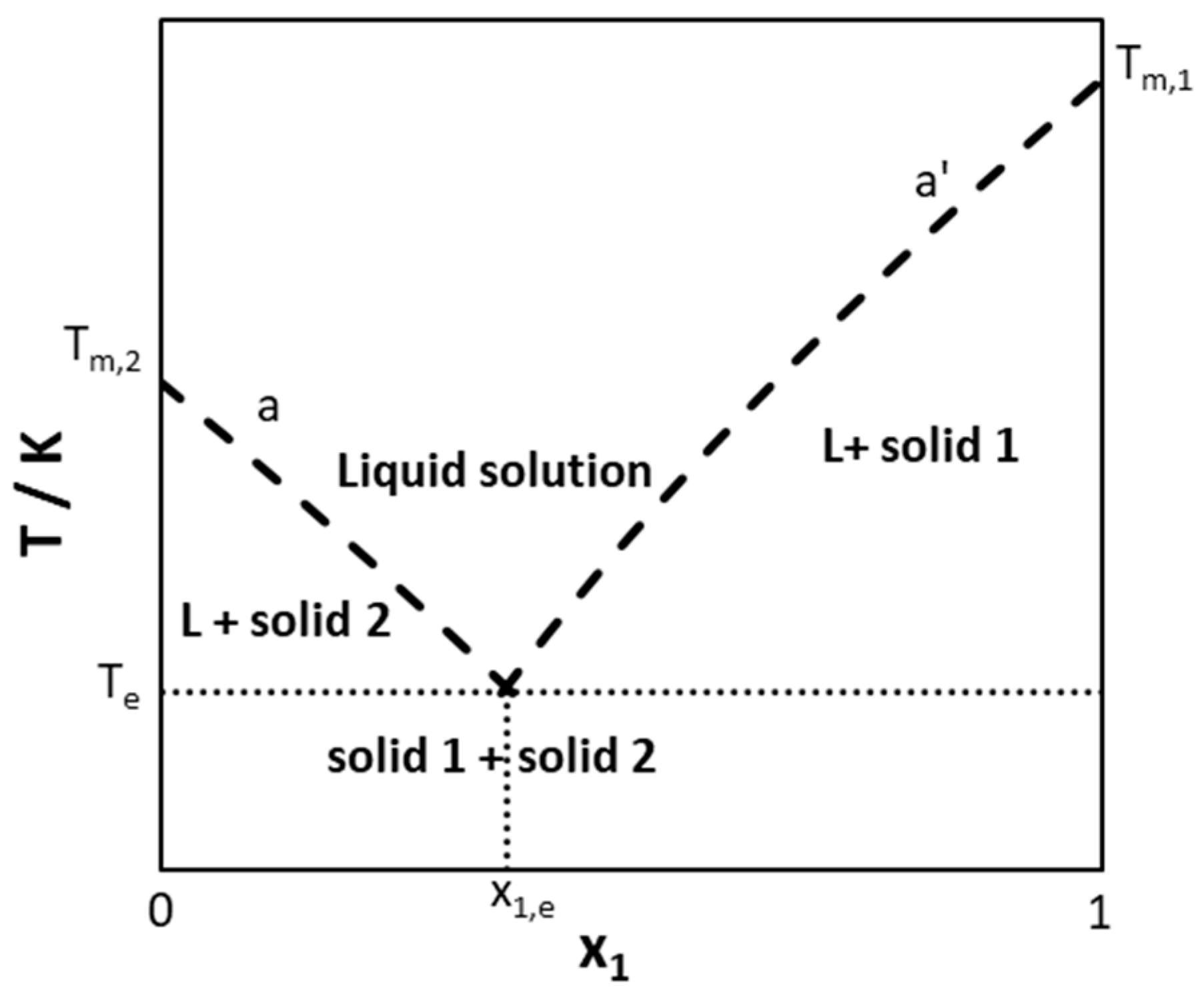
From mungfali.com
Types Of Equilibrium Chemistry Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: There are many examples of pairs of compounds that show this kind of behavior. The curves represent the points. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: One combination is sodium. Explain Solid Liquid Equilibrium With An Example.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Explain Solid Liquid Equilibrium With An Example In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. The curves represent the points. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: There are many examples of pairs of compounds that show this kind. Explain Solid Liquid Equilibrium With An Example.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this kind of behavior. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The curves represent the points. In this lesson,. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this kind of behavior. The curves represent the points. It is a familiar fact that pure substances tend to exist in one of three distinct states: One combination is sodium and potassium,. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
solid liquid equilibrium equilibrium part 2 class 11 Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). It is a familiar fact that pure substances tend to exist in one of three distinct states: The curves represent the points. There are many examples of pairs of. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
SolidLiquid Equilibrium YouTube Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. In this lesson, we develop the consequences of this law to. Explain Solid Liquid Equilibrium With An Example.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact that pure substances tend to exist in one of three distinct states: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Explain Solid Liquid Equilibrium With An Example Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is. Explain Solid Liquid Equilibrium With An Example.
From slidetodoc.com
LiquidLiquid Equilibrium Ternary System Most practical situations involving Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this kind of behavior. It is a familiar fact that pure substances tend to exist in one of three distinct states: The curves represent the points. One combination is sodium and potassium,. Explain Solid Liquid Equilibrium With An Example.
From www.researchgate.net
Solidliquid equilibrium diagram of therapeutic eutectic building Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact that pure substances tend to exist in one of three distinct states: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. The curves. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free Explain Solid Liquid Equilibrium With An Example In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are many examples of pairs of compounds that show this kind of behavior. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. Consider an equilibrium between a. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT The Concept of Equilibrium PowerPoint Presentation, free download Explain Solid Liquid Equilibrium With An Example The curves represent the points. There are many examples of pairs of compounds that show this kind of behavior. It is a familiar fact that pure substances tend to exist in one of three distinct states: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Explain Solid Liquid Equilibrium With An Example The curves represent the points. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT Unit 2 Solids, Liquids, Equilibrium, Solubility PowerPoint Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact that pure substances tend to exist in one of three distinct states: The curves represent the points. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the. Explain Solid Liquid Equilibrium With An Example.
From www.showme.com
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are many examples of pairs of compounds that show this. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact that pure substances tend to exist in one of three distinct states: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. The curves. Explain Solid Liquid Equilibrium With An Example.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Explain Solid Liquid Equilibrium With An Example In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are many examples of pairs of compounds that show this kind of behavior. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact. Explain Solid Liquid Equilibrium With An Example.
From learncheme.com
ssleequilibrium LearnChemE Explain Solid Liquid Equilibrium With An Example The curves represent the points. In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. It is a familiar fact that pure substances tend to exist in one of three distinct states: One combination is sodium and potassium, which form a compound (na2k n a 2 k). Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: The curves represent the points. There are many examples of pairs of compounds that show this kind of behavior. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
⚗️ Writing Equilibrium Expressions for Reactions Involving a Solid or a Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: There are many examples of pairs of compounds that. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and. Explain Solid Liquid Equilibrium With An Example.
From www.numerade.com
SOLVED The figure below is the solidliquid phase diagram for the Explain Solid Liquid Equilibrium With An Example Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. It is a familiar fact that pure substances tend to exist in one of three distinct states: In this lesson, we develop. Explain Solid Liquid Equilibrium With An Example.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Explain Solid Liquid Equilibrium With An Example In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are many examples of pairs of compounds that show this kind of behavior. The curves represent the points. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the. Explain Solid Liquid Equilibrium With An Example.
From www.researchgate.net
Simple Binary SolidLiquid Equilibrium Diagram Download Scientific Explain Solid Liquid Equilibrium With An Example There are many examples of pairs of compounds that show this kind of behavior. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). In this lesson, we develop the consequences of this law to answer the very practical. Explain Solid Liquid Equilibrium With An Example.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. There are. Explain Solid Liquid Equilibrium With An Example.
From slidetodoc.com
LiquidLiquid Equilibrium Ternary System Most practical situations involving Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). Consider an equilibrium between a crystalline salt (or other kind of ionic. Explain Solid Liquid Equilibrium With An Example.
From www.slideserve.com
PPT Phase Equilibrium II Two Component System PowerPoint Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase. Explain Solid Liquid Equilibrium With An Example.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Explain Solid Liquid Equilibrium With An Example Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition. Explain Solid Liquid Equilibrium With An Example.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Explain Solid Liquid Equilibrium With An Example The curves represent the points. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: It is a familiar fact that pure substances tend to exist in one of three distinct states: One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable. Explain Solid Liquid Equilibrium With An Example.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There are many examples of pairs. Explain Solid Liquid Equilibrium With An Example.
From ecampusontario.pressbooks.pub
Chemical Equilibrium First Year General Chemistry Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: There are many examples of pairs of compounds that show this kind of behavior. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen &. Explain Solid Liquid Equilibrium With An Example.
From www.scribd.com
Solid Liquid Equilibrium Phase (Matter) Phase Diagram Explain Solid Liquid Equilibrium With An Example The curves represent the points. One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. There. Explain Solid Liquid Equilibrium With An Example.
From www.researchgate.net
Phase diagram of solid liquid equilibrium in the system H 2 O FeSO Explain Solid Liquid Equilibrium With An Example Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. The curves represent the points. It is a familiar fact that pure substances tend to exist in one of. Explain Solid Liquid Equilibrium With An Example.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Explain Solid Liquid Equilibrium With An Example One combination is sodium and potassium, which form a compound (na2k n a 2 k) that is unstable in the liquid phase and so it melts incongruently (rossen & bleiswijk, 1912). In this lesson, we develop the consequences of this law to answer the very practical question of how an existing equilibrium composition is. Consider an equilibrium between a crystalline. Explain Solid Liquid Equilibrium With An Example.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Explain Solid Liquid Equilibrium With An Example It is a familiar fact that pure substances tend to exist in one of three distinct states: Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: There are many examples of pairs of compounds that show this kind of behavior. Figure 1 illustrates the temperatures and pressures at which. Explain Solid Liquid Equilibrium With An Example.