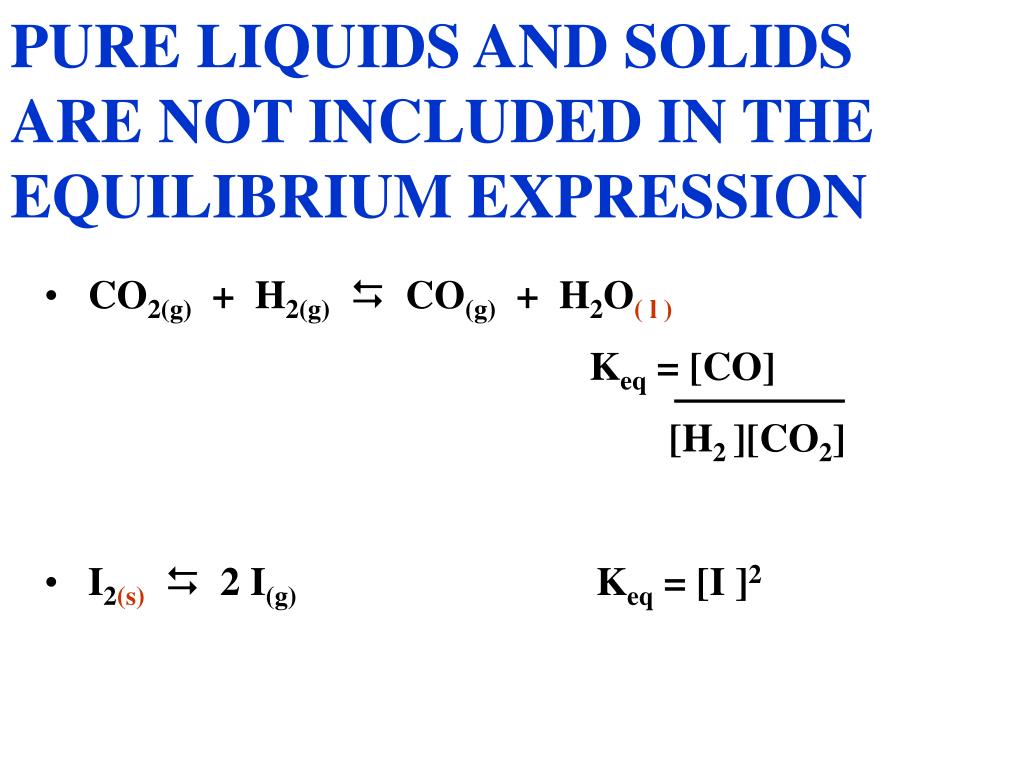
Solids Liquids Equilibrium Constant . Concentrations enter the equilibrium constant. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). [op] why are solids and liquids not included in the equilibrium constant? They are, in a way. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. We can readily derive a relation between \(k\subs{s}\) and the. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the.
from www.slideserve.com
The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. Concentrations enter the equilibrium constant. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. We can readily derive a relation between \(k\subs{s}\) and the. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. They are, in a way. [op] why are solids and liquids not included in the equilibrium constant?
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID
Solids Liquids Equilibrium Constant The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. They are, in a way. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. Concentrations enter the equilibrium constant. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. [op] why are solids and liquids not included in the equilibrium constant? The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. We can readily derive a relation between \(k\subs{s}\) and the.
From slideplayer.com
The Equilibrium Constant, Heterogeneous Equilibria, The Equilibrium Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. They are, in a way. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. In equations 6 through 10, you saw how the constant concentration of. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation ID3889861 Solids Liquids Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? We can readily derive a relation between \(k\subs{s}\) and the. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. Concentrations enter the equilibrium constant. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Section 8.2—Equilibrium Constant PowerPoint Presentation, free Solids Liquids Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. Concentrations enter the equilibrium constant. In equations 6 through. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Solids Liquids Equilibrium Constant My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. They are, in a way. Concentrations enter the equilibrium constant. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. Learn how to. Solids Liquids Equilibrium Constant.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Solids Liquids Equilibrium Constant In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. They are, in a way. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. We can readily. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. They are, in a way. The. Solids Liquids Equilibrium Constant.
From slidetodoc.com
14 4 14 7 Equilibrium Constant in Terms Solids Liquids Equilibrium Constant They are, in a way. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The equilibrium constant of pressure gives the ratio of pressure of. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. Concentrations enter the equilibrium constant. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure. Solids Liquids Equilibrium Constant.
From slideplayer.com
Introduction to the Equilibrium Constant ppt download Solids Liquids Equilibrium Constant Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. Concentrations enter the equilibrium constant. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility. Solids Liquids Equilibrium Constant.
From www.youtube.com
Week 7 2. How to handle solids and liquids when writing an Solids Liquids Equilibrium Constant They are, in a way. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. [op] why are solids and liquids not included in the equilibrium constant? When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. In equations 6 through. Solids Liquids Equilibrium Constant.
From slideplayer.com
Equilibrium. ppt download Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. [op] why are solids and liquids not included in the equilibrium constant? They are, in a way. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The thermodynamic equilibrium constant. Solids Liquids Equilibrium Constant.
From slideplayer.com
Chemical Equilibrium The state where the concentrations of all Solids Liquids Equilibrium Constant In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. We can readily derive a relation between \(k\subs{s}\) and the. [op] why are solids and liquids not included in the equilibrium constant? The thermodynamic equilibrium constant for. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Unit 2 Liquids and solids, solubility, equilibrium PowerPoint Solids Liquids Equilibrium Constant In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The equilibrium constant of pressure gives the. Solids Liquids Equilibrium Constant.
From www.numerade.com
SOLVED\( \mathrm{} \) \( \mathrm{} \) Solids Liquids Equilibrium Constant We can readily derive a relation between \(k\subs{s}\) and the. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. [op] why are solids and liquids not included in the equilibrium constant? The equilibrium constant of pressure. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT The Concept of Equilibrium PowerPoint Presentation, free download Solids Liquids Equilibrium Constant Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. We can readily derive a relation between \(k\subs{s}\) and the. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). Concentrations enter the equilibrium constant. In equations 6 through 10, you saw how the constant concentration. Solids Liquids Equilibrium Constant.
From slideplayer.com
Equilibrium Constant Kc = [C]c[D]d [A]a[B]b ppt download Solids Liquids Equilibrium Constant My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. They are, in a way. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. We can readily derive a relation between \(k\subs{s}\) and the. [op]. Solids Liquids Equilibrium Constant.
From www.numerade.com
Explain why pure liquids and solids can be ignored when writing the Solids Liquids Equilibrium Constant Concentrations enter the equilibrium constant. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again,. Solids Liquids Equilibrium Constant.
From slidetodoc.com
Chemical Equilibrium The Concept of Equilibrium No chemical Solids Liquids Equilibrium Constant We can readily derive a relation between \(k\subs{s}\) and the. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. They are, in a way. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be. Solids Liquids Equilibrium Constant.
From present5.com
Chapter 3 Chemical Equilibrium Reactions Irreversible Reversible The Solids Liquids Equilibrium Constant They are, in a way. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant. We can readily. Solids Liquids Equilibrium Constant.
From slideplayer.com
Equilibrium Keq. ppt download Solids Liquids Equilibrium Constant The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. Concentrations enter the equilibrium constant. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility. Solids Liquids Equilibrium Constant.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solids Liquids Equilibrium Constant The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. They are, in a way. Concentrations enter the equilibrium constant. [op] why are solids and liquids not included in the equilibrium constant? We can readily derive a relation between \(k\subs{s}\) and the. The thermodynamic equilibrium constant for. Solids Liquids Equilibrium Constant.
From slideplayer.com
CHEMICAL EQUILIBRIUM Many chemical reactions run to completion ppt Solids Liquids Equilibrium Constant The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. Concentrations enter the equilibrium constant. They are, in a way. We can readily. Solids Liquids Equilibrium Constant.
From www.youtube.com
15.5 Heterogeneous Equilibria Reactions Involving a Solid or a Liquid Solids Liquids Equilibrium Constant Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. They are, in a way. [op] why are solids and liquids not included in the equilibrium constant? In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can. Solids Liquids Equilibrium Constant.
From www.youtube.com
⚗️ Writing Equilibrium Expressions for Reactions Involving a Solid or a Solids Liquids Equilibrium Constant Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. They are, in a way. Concentrations enter the equilibrium constant. In equations 6 through 10, you saw how. Solids Liquids Equilibrium Constant.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Solids Liquids Equilibrium Constant We can readily derive a relation between \(k\subs{s}\) and the. Concentrations enter the equilibrium constant. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Unit 9 and Equilibrium PowerPoint Presentation, free Solids Liquids Equilibrium Constant My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. We can readily derive a relation between \(k\subs{s}\) and the. The thermodynamic equilibrium constant for this. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2633301 Solids Liquids Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? We can readily derive a relation between \(k\subs{s}\) and the. They are, in a way. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the equilibrium constant.. Solids Liquids Equilibrium Constant.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Solids Liquids Equilibrium Constant My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. They are, in a way. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions,. Solids Liquids Equilibrium Constant.
From www.youtube.com
Equilibrium Constants Kc, Kp, solids and liquids (106 Module 1 Video 2 Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). They are, in a way. In equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium Constant PowerPoint Presentation, free download ID Solids Liquids Equilibrium Constant When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). Concentrations enter the equilibrium constant. My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure. Solids Liquids Equilibrium Constant.
From slidetodoc.com
CHEMICAL EQUILIBRIUM Many chemical reactions run to completion Solids Liquids Equilibrium Constant We can readily derive a relation between \(k\subs{s}\) and the. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. They are, in a way. Concentrations enter the. Solids Liquids Equilibrium Constant.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Solids Liquids Equilibrium Constant We can readily derive a relation between \(k\subs{s}\) and the. Concentrations enter the equilibrium constant. [op] why are solids and liquids not included in the equilibrium constant? Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The equilibrium constant of pressure gives the ratio of pressure of products over reactants. Solids Liquids Equilibrium Constant.
From www.reddit.com
Isn’t this supposed to be no shift since liquids and solids are not Solids Liquids Equilibrium Constant The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that is at equilibrium (again, the. They are, in a way. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and. Solids Liquids Equilibrium Constant.
From www.youtube.com
Pure solids & Pure liquids can be ignored while writing expression for Solids Liquids Equilibrium Constant My explanation is that the molarity (concentration and also the activity in case of an ideal solution) of a pure liquid (or solid) won't. When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities. The equilibrium constant of pressure gives the ratio of pressure of products over reactants for a. Solids Liquids Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium Basic Concepts PowerPoint Presentation, free download Solids Liquids Equilibrium Constant Concentrations enter the equilibrium constant. They are, in a way. We can readily derive a relation between \(k\subs{s}\) and the. Learn how to calculate equilibrium constants for homogeneous and heterogeneous reactions, and how to use them to predict equilibrium. The thermodynamic equilibrium constant for this kind of equilibrium is called a solubility product, \(k\subs{s}\). In equations 6 through 10, you. Solids Liquids Equilibrium Constant.