Natural Gas Burning Chemical Equation . Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The equation of combustion is then pretty simple: A complete combustion is a. The methane gas burns with a clear blue flame. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat.
from www.youtube.com
Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). A complete combustion is a. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. The methane gas burns with a clear blue flame. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The equation of combustion is then pretty simple: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units.
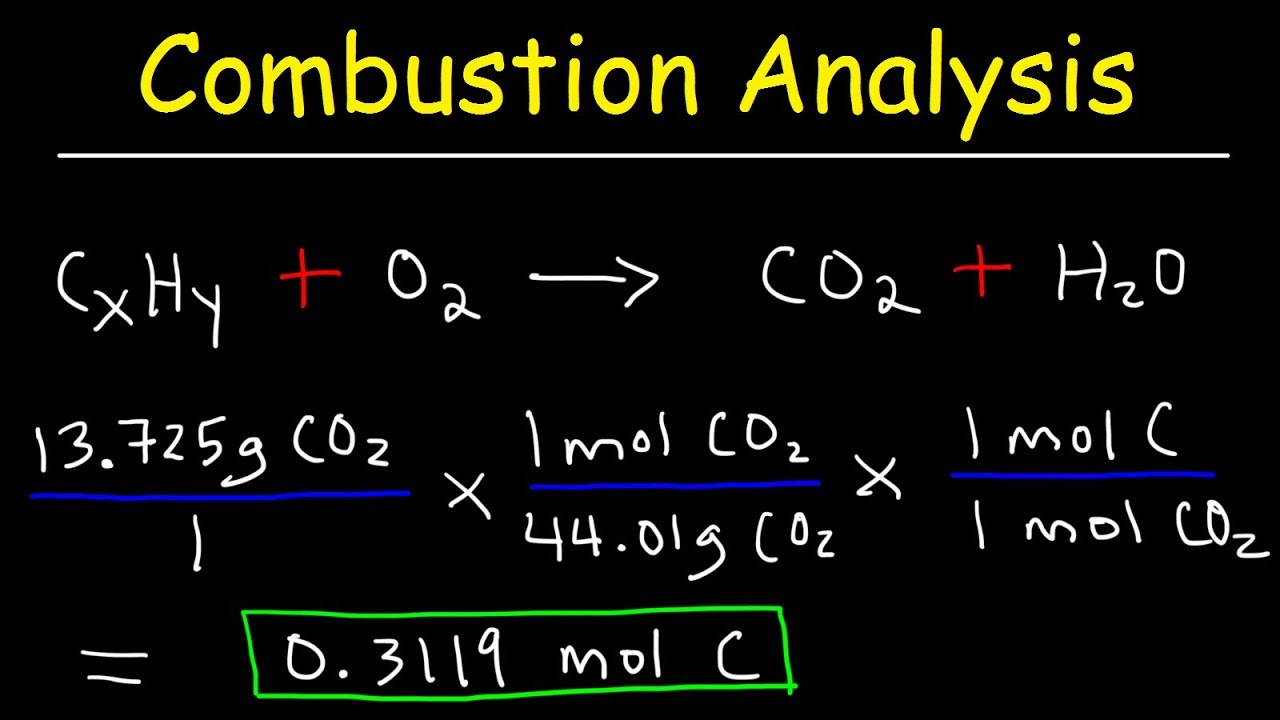
Introduction to Combustion Analysis, Empirical Formula & Molecular
Natural Gas Burning Chemical Equation The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. A complete combustion is a. The equation of combustion is then pretty simple: The methane gas burns with a clear blue flame. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the.
From methanegaskimawashi.blogspot.com
Methane Gas Methane Gas And Oxygen Balanced Equation Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Methane + oxygen carbon dioxide + water + energy. Natural Gas Burning Chemical Equation.
From www.chegg.com
Solved 2. Combustion Stoichiometry and Properties of a Gas Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. The equation of combustion is then pretty simple: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. Combustion is a reaction between a hydrocarbon fuel (e.g.,. Natural Gas Burning Chemical Equation.
From mungfali.com
Methane Balanced Equation Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create. Natural Gas Burning Chemical Equation.
From www.slideserve.com
PPT Combustion PowerPoint Presentation ID1992427 Natural Gas Burning Chemical Equation The equation of combustion is then pretty simple: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The methane gas burns with a clear blue flame.. Natural Gas Burning Chemical Equation.
From www.wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Natural Gas Burning Chemical Equation Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. The equation of combustion is then pretty simple: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. The methane gas. Natural Gas Burning Chemical Equation.
From www.grc.nasa.gov
Combustion Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The fuel input is 12 atomic mass units,. Natural Gas Burning Chemical Equation.
From sciencenotes.org
Combustion Reaction Definition and Examples Natural Gas Burning Chemical Equation The equation of combustion is then pretty simple: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the.. Natural Gas Burning Chemical Equation.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. The equation of combustion is then pretty simple: A complete combustion is a. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. On burning of natural gas i.e., combustion of. Natural Gas Burning Chemical Equation.
From bdteletalk.com
Equation For The Combustion Of Methane Natural Gas Burning Chemical Equation Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The equation of combustion is then pretty simple: Combustion is. Natural Gas Burning Chemical Equation.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation ID1996069 Natural Gas Burning Chemical Equation A complete combustion is a. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The equation of combustion is then pretty simple: The methane gas burns with a clear blue flame. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create. Natural Gas Burning Chemical Equation.
From preparatorychemistry.com
Combustion Analysis Natural Gas Burning Chemical Equation The equation of combustion is then pretty simple: A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. The methane gas burns with a clear blue flame. Combustion is a reaction. Natural Gas Burning Chemical Equation.
From www.saubhaya.com
Chemical Makeup Of Natural Gas Saubhaya Makeup Natural Gas Burning Chemical Equation A complete combustion is a. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. The methane gas burns with a clear blue flame. Stoichiometric. Natural Gas Burning Chemical Equation.
From www.youtube.com
Material Balances on Complete Combustion of Methane YouTube Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The equation of combustion is then pretty simple: A complete combustion is a. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water. Natural Gas Burning Chemical Equation.
From organicvsa.weebly.com
Combustion equations 4 chemical equations and stoichiometry organicvsa Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. The equation of combustion is then pretty simple: On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2. Natural Gas Burning Chemical Equation.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create. Natural Gas Burning Chemical Equation.
From www.youtube.com
Introduction to Combustion Analysis, Empirical Formula & Molecular Natural Gas Burning Chemical Equation A complete combustion is a. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood,. Natural Gas Burning Chemical Equation.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Natural Gas Burning Chemical Equation On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The equation of combustion is then pretty simple: The methane gas burns with a clear blue flame. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Methane. Natural Gas Burning Chemical Equation.
From www.youtube.com
How to Balance C2H6 + O2 = CO2 + H2O (Ethane Combustion Reaction) YouTube Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. A complete combustion is a. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The equation of combustion is then pretty simple: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon. Natural Gas Burning Chemical Equation.
From studylib.net
20 Write a balanced equation for the complete combustion of each Natural Gas Burning Chemical Equation The methane gas burns with a clear blue flame. The equation of combustion is then pretty simple: The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. A complete combustion is a. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co. Natural Gas Burning Chemical Equation.
From www.edwardtdodge.com
Reverse Combustion Equation Edward T. Dodge Natural Gas Burning Chemical Equation Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The methane gas burns with a clear blue. Natural Gas Burning Chemical Equation.
From www.youtube.com
AQA A Level Chemistry Organic Chemistry Combustion YouTube Natural Gas Burning Chemical Equation Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. A complete combustion is a. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon. Natural Gas Burning Chemical Equation.
From www.researchgate.net
Typical natural gas composition Download Table Natural Gas Burning Chemical Equation On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. Combustion is a. Natural Gas Burning Chemical Equation.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Natural Gas Burning Chemical Equation A complete combustion is a. The equation of combustion is then pretty simple: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. On burning of natural gas i.e., combustion of methane leads to oxidation reaction. Natural Gas Burning Chemical Equation.
From www.revisechemistry.uk
Interpreting and interacting with Earth systems OCR Gateway C6 Natural Gas Burning Chemical Equation On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The methane gas burns with a clear blue flame. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and. Natural Gas Burning Chemical Equation.
From www.scribd.com
Chemical Composition of Natural Gas Natural Gas Combustion Natural Gas Burning Chemical Equation Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The. Natural Gas Burning Chemical Equation.
From studylib.net
COMBUSTION of PROPANE Natural Gas Burning Chemical Equation A complete combustion is a. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen. Natural Gas Burning Chemical Equation.
From methanegaskimawashi.blogspot.com
Methane Gas Combustion Of Methane Gas Equation Natural Gas Burning Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. On. Natural Gas Burning Chemical Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Complete Combustion Of Gasoline Natural Gas Burning Chemical Equation Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. The methane gas. Natural Gas Burning Chemical Equation.
From www.youtube.com
Fuels and Combustion Part 6 (Important Combustion equations) YouTube Natural Gas Burning Chemical Equation The equation of combustion is then pretty simple: On burning of natural gas i.e., combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the. The methane gas burns with a clear blue flame. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat.. Natural Gas Burning Chemical Equation.
From www.saubhaya.com
Chemical Makeup Of Natural Gas Saubhaya Makeup Natural Gas Burning Chemical Equation A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The methane gas burns with a clear blue flame. The equation of combustion is then pretty simple: Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water. Natural Gas Burning Chemical Equation.
From www.youtube.com
Balancing equations combustion Reactions meriSTEM YouTube Natural Gas Burning Chemical Equation Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and. Natural Gas Burning Chemical Equation.
From www.youtube.com
Balancing Combustion Reactions Chemistry Tutorial YouTube Natural Gas Burning Chemical Equation Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co. Natural Gas Burning Chemical Equation.
From mungfali.com
Complete Combustion Of Methane Equation Natural Gas Burning Chemical Equation A complete combustion is a. Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l). Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing. Natural Gas Burning Chemical Equation.
From www.tessshebaylo.com
Chemical Equation For Burning Natural Gas Tessshebaylo Natural Gas Burning Chemical Equation Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The fuel input is 12 atomic mass units, the co2 output is 44 atomic mass units. The equation of combustion is then pretty simple: Methane + oxygen carbon dioxide + water + energy ch 4 (g) + 2o 2(g) co 2(g) + 2h 2 o (l).. Natural Gas Burning Chemical Equation.
From www.tessshebaylo.com
Chemical Equation For Burning Of Natural Gas Tessshebaylo Natural Gas Burning Chemical Equation Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Stoichiometric or theoretical combustion is the ideal combustion process where fuel. Natural Gas Burning Chemical Equation.