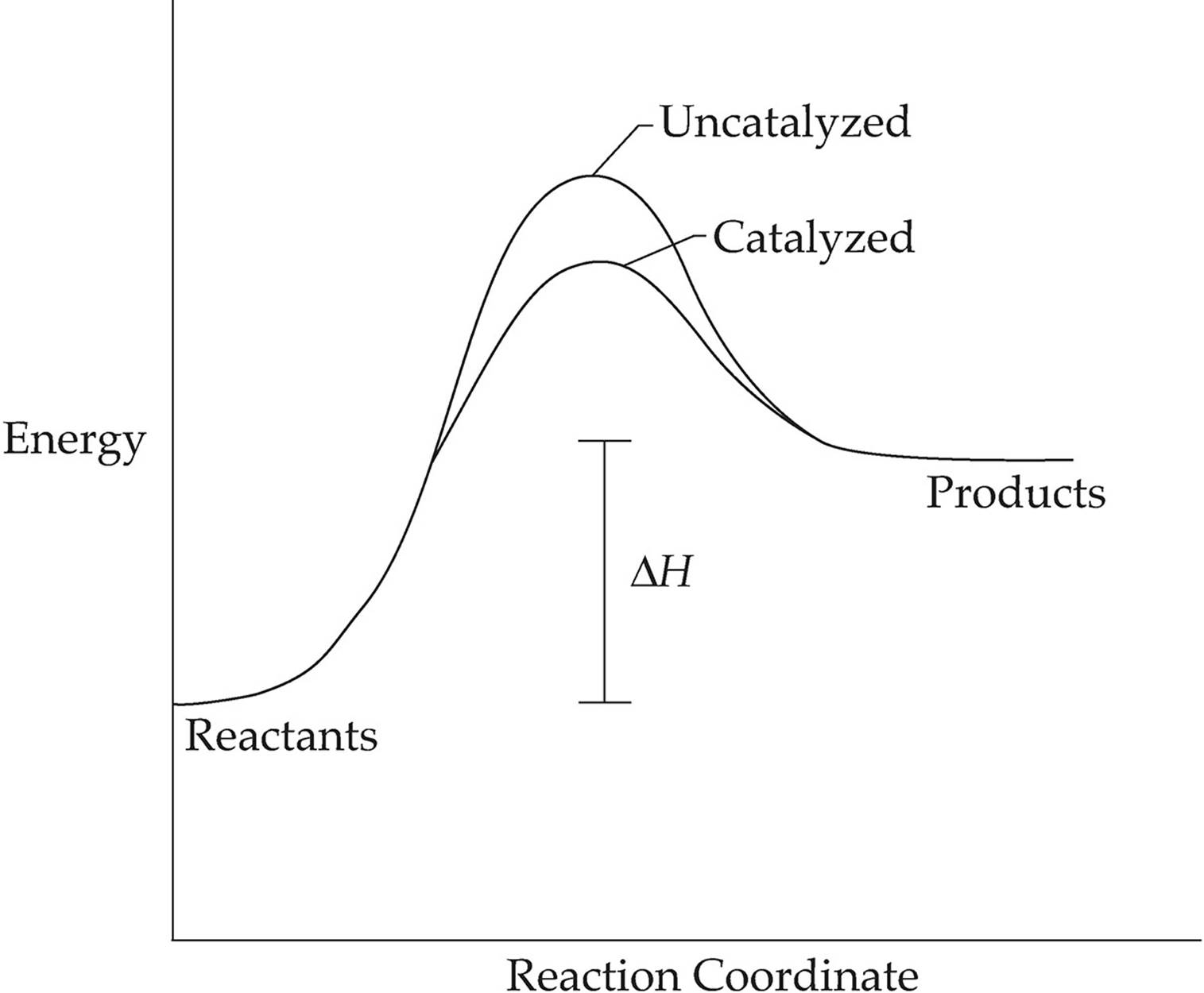
Do Catalysts Lower Activation Energy Of Reverse Reaction . In addition, the catalyst is regenerated in the process. A catalyst lowers the activation energy by changing the transition state of the reaction. In other words, to move the activation energy to the left on the graph:. Several reactions that are thermodynamically. Catalysts do not just reduce the energy barrier, but induced a completely. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst speeds up the rate of a reaction by lowering the activation energy; See below for the effects of an enzyme on activation energy. The idea is that the activation energies is lowered for both the forward and backward reactions. The catalyst provides a different reaction path with a lower activation energy. (2) a catalyst can open a new reaction pathway with lower activation energy. One possible way of doing this is to provide an alternative way for. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. It does it by providing a partial bond which stabilises the transition state and compensates for part of the.
from schoolbag.info
Several reactions that are thermodynamically. (2) a catalyst can open a new reaction pathway with lower activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. The idea is that the activation energies is lowered for both the forward and backward reactions. The catalyst provides a different reaction path with a lower activation energy. A catalyst lowers the activation energy by changing the transition state of the reaction. In addition, the catalyst is regenerated in the process. See below for the effects of an enzyme on activation energy. In other words, to move the activation energy to the left on the graph:. It does it by providing a partial bond which stabilises the transition state and compensates for part of the.
A catalyst speeds up a reaction by providing the reactants with an
Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. See below for the effects of an enzyme on activation energy. In other words, to move the activation energy to the left on the graph:. It does it by providing a partial bond which stabilises the transition state and compensates for part of the. The catalyst provides a different reaction path with a lower activation energy. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. The idea is that the activation energies is lowered for both the forward and backward reactions. Several reactions that are thermodynamically. Catalysts do not just reduce the energy barrier, but induced a completely. In addition, the catalyst is regenerated in the process. (2) a catalyst can open a new reaction pathway with lower activation energy. One possible way of doing this is to provide an alternative way for. To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst lowers the activation energy by changing the transition state of the reaction.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst speeds up the rate of a reaction by lowering the activation energy; (2) a catalyst can open a new reaction pathway with lower activation energy. Several reactions that are thermodynamically. See below for the effects of an enzyme on activation energy. In other words, to move the activation energy to the left on the graph:. The idea is. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Do Catalysts Lower Activation Energy Of Reverse Reaction The idea is that the activation energies is lowered for both the forward and backward reactions. A catalyst lowers the activation energy by changing the transition state of the reaction. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In addition, the catalyst is regenerated in. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From brainly.com
Which of the following statements about catalysts is false? A catalyst Do Catalysts Lower Activation Energy Of Reverse Reaction Several reactions that are thermodynamically. It does it by providing a partial bond which stabilises the transition state and compensates for part of the. See below for the effects of an enzyme on activation energy. The idea is that the activation energies is lowered for both the forward and backward reactions. (2) a catalyst can open a new reaction pathway. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.globalspec.com
Catalysts and Initiators Information Engineering360 Do Catalysts Lower Activation Energy Of Reverse Reaction One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy to the left on the graph:. The catalyst provides a different reaction path with a lower activation energy. See below for the effects of an enzyme on activation energy.. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Do Catalysts Lower Activation Energy Of Reverse Reaction In addition, the catalyst is regenerated in the process. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. One possible way of doing this is to provide an alternative way for. Several reactions that are thermodynamically. Catalysts do not just reduce the energy barrier, but induced. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From dxojlxtmb.blob.core.windows.net
How Do Enzymes Act As Biological Catalysts (Choose 3) at Robert Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; It does it by providing a partial bond which stabilises the transition state and compensates for part of the. One possible way of doing this is to provide an alternative way for the reaction to. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From gioyfzlvn.blob.core.windows.net
Which Catalysts Lower Activation Energy at Ronald Lerner blog Do Catalysts Lower Activation Energy Of Reverse Reaction Several reactions that are thermodynamically. To increase the rate of a reaction you need to increase the number of successful collisions. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. One possible way of doing this is to provide an alternative way for. Catalysts do not just reduce the energy barrier, but induced a completely. In other. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? Do Catalysts Lower Activation Energy Of Reverse Reaction The idea is that the activation energies is lowered for both the forward and backward reactions. See below for the effects of an enzyme on activation energy. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. The catalyst provides a different reaction path with a lower activation energy. In other words, to move the activation energy to. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst lowers the activation energy by changing the transition state of the reaction. The catalyst provides a different reaction path with a lower activation energy. In other words, to move the activation energy to the left on the graph:. (2) a catalyst can open a new reaction pathway with lower activation energy. The reaction then goes through a different. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Do Catalysts Lower Activation Energy Of Reverse Reaction One possible way of doing this is to provide an alternative way for. A catalyst lowers the activation energy by changing the transition state of the reaction. Several reactions that are thermodynamically. (2) a catalyst can open a new reaction pathway with lower activation energy. Catalysts do not just reduce the energy barrier, but induced a completely. It does it. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Do Catalysts Lower Activation Energy Of Reverse Reaction The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. It does it by providing a partial bond which stabilises the transition state and compensates for part of the. (2) a catalyst can open a new reaction pathway with lower activation energy. A catalyst lowers the activation energy by changing the transition state of the reaction. One possible. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Do Catalysts Lower Activation Energy Of Reverse Reaction Catalysts do not just reduce the energy barrier, but induced a completely. A catalyst speeds up the rate of a reaction by lowering the activation energy; (2) a catalyst can open a new reaction pathway with lower activation energy. The catalyst provides a different reaction path with a lower activation energy. It does it by providing a partial bond which. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Do Catalysts Lower Activation Energy Of Reverse Reaction Several reactions that are thermodynamically. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; In other words, to move the activation energy to the left on the graph:. A catalyst lowers the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From circuitestrilhatc6.z13.web.core.windows.net
Energy Diagram Chemdraw Do Catalysts Lower Activation Energy Of Reverse Reaction One possible way of doing this is to provide an alternative way for. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy to the left on the graph:. The catalyst provides a different reaction path with a lower. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Do Catalysts Lower Activation Energy Of Reverse Reaction In other words, to move the activation energy to the left on the graph:. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts do not just reduce the energy barrier, but induced a completely. One possible way of doing this is to provide an alternative way for the reaction to happen which. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst lowers the activation energy by changing the transition state of the reaction. The catalyst provides a different reaction path with a lower activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. Several reactions that are thermodynamically. In other words, to move the activation energy to the left on the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.youtube.com
Enzyme Basics & How Do They Lower Activation Energy? YouTube Do Catalysts Lower Activation Energy Of Reverse Reaction The catalyst provides a different reaction path with a lower activation energy. In other words, to move the activation energy to the left on the graph:. (2) a catalyst can open a new reaction pathway with lower activation energy. Several reactions that are thermodynamically. A catalyst speeds up the rate of a reaction by lowering the activation energy; In addition,. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 Do Catalysts Lower Activation Energy Of Reverse Reaction To increase the rate of a reaction you need to increase the number of successful collisions. The catalyst provides a different reaction path with a lower activation energy. See below for the effects of an enzyme on activation energy. In other words, to move the activation energy to the left on the graph:. (2) a catalyst can open a new. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Do Catalysts Lower Activation Energy Of Reverse Reaction (2) a catalyst can open a new reaction pathway with lower activation energy. Catalysts do not just reduce the energy barrier, but induced a completely. The catalyst provides a different reaction path with a lower activation energy. A catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that are thermodynamically. In addition, the catalyst. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Do Catalysts Lower Activation Energy Of Reverse Reaction It does it by providing a partial bond which stabilises the transition state and compensates for part of the. The idea is that the activation energies is lowered for both the forward and backward reactions. Several reactions that are thermodynamically. See below for the effects of an enzyme on activation energy. The reaction then goes through a different pathway/mechanism than. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.sliderbase.com
Catalysis Presentation Chemistry Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst lowers the activation energy by changing the transition state of the reaction. One possible way of doing this is to provide an alternative way for. See below for the effects of an enzyme on activation energy. It does it by providing a partial bond which stabilises the transition state and compensates for part of the. In addition, the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. Several reactions that are thermodynamically. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. The catalyst provides a different reaction path with a. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From slidetodoc.com
Enzymes are proteins that act as biological catalysts Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. In other words, to move the activation energy to the left on the graph:. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. Catalysts do not just reduce the energy barrier, but induced a completely.. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Do Catalysts Lower Activation Energy Of Reverse Reaction Several reactions that are thermodynamically. A catalyst speeds up the rate of a reaction by lowering the activation energy; Catalysts do not just reduce the energy barrier, but induced a completely. In addition, the catalyst is regenerated in the process. In other words, to move the activation energy to the left on the graph:. One possible way of doing this. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Do Catalysts Lower Activation Energy Of Reverse Reaction One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. It does it by providing a partial bond which stabilises the transition state and compensates for part of the. One possible way of doing this is to provide an alternative way for. A catalyst speeds up the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From fyoshifvq.blob.core.windows.net
Enzymes Chemical Barrier at Filiberto Saunders blog Do Catalysts Lower Activation Energy Of Reverse Reaction The idea is that the activation energies is lowered for both the forward and backward reactions. See below for the effects of an enzyme on activation energy. One possible way of doing this is to provide an alternative way for. Catalysts do not just reduce the energy barrier, but induced a completely. In addition, the catalyst is regenerated in the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Do Catalysts Lower Activation Energy Of Reverse Reaction In other words, to move the activation energy to the left on the graph:. In addition, the catalyst is regenerated in the process. One possible way of doing this is to provide an alternative way for. (2) a catalyst can open a new reaction pathway with lower activation energy. Catalysts do not just reduce the energy barrier, but induced a. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Endergonic and Exergonic Reactions PowerPoint Presentation ID Do Catalysts Lower Activation Energy Of Reverse Reaction It does it by providing a partial bond which stabilises the transition state and compensates for part of the. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. The catalyst provides a different reaction path with a lower activation energy. One possible way of doing this. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Do Catalysts Lower Activation Energy Of Reverse Reaction One possible way of doing this is to provide an alternative way for. Several reactions that are thermodynamically. The catalyst provides a different reaction path with a lower activation energy. See below for the effects of an enzyme on activation energy. It does it by providing a partial bond which stabilises the transition state and compensates for part of the.. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. Catalysts do not just reduce the energy barrier, but induced a completely. In addition, the catalyst is regenerated in the process. One possible way of doing this is to provide an alternative way for. It does it by providing a partial bond which stabilises the transition state and compensates. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Do Catalysts Lower Activation Energy Of Reverse Reaction In other words, to move the activation energy to the left on the graph:. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. See below for the effects of an enzyme on activation energy. The catalyst provides a different reaction path with a lower activation energy.. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Enzymes and Enzyme Activity Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst lowers the activation energy by changing the transition state of the reaction. The catalyst provides a different reaction path with a lower activation energy. One possible way of doing this is to provide an alternative way for. The reaction then goes through a different pathway/mechanism than the uncatalyzed reaction. To increase the rate of a reaction you need. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Do Catalysts Lower Activation Energy Of Reverse Reaction See below for the effects of an enzyme on activation energy. Several reactions that are thermodynamically. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. The catalyst provides a different reaction path with a lower activation energy. A catalyst lowers the activation energy by changing the. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst speeds up the rate of a reaction by lowering the activation energy; In addition, the catalyst is regenerated in the process. The idea is that the activation energies is lowered for both the forward and backward reactions. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. Do Catalysts Lower Activation Energy Of Reverse Reaction.
From exodjtdpy.blob.core.windows.net
Enzymes Raise The Activation Energy Of A Reaction at Sean Ferguson blog Do Catalysts Lower Activation Energy Of Reverse Reaction A catalyst lowers the activation energy by changing the transition state of the reaction. A catalyst speeds up the rate of a reaction by lowering the activation energy; To increase the rate of a reaction you need to increase the number of successful collisions. In other words, to move the activation energy to the left on the graph:. Catalysts do. Do Catalysts Lower Activation Energy Of Reverse Reaction.