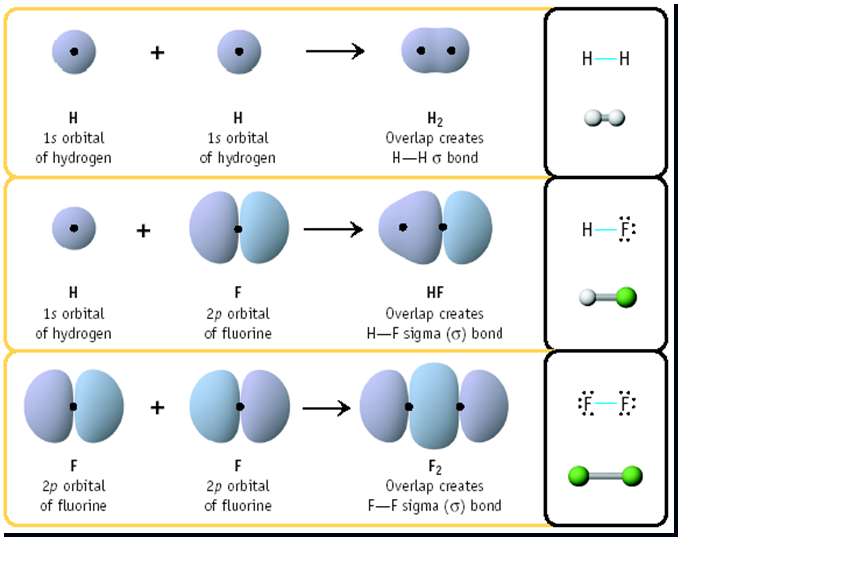
What Is A Molecular Orbital Model . A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Using molecular orbital theory, we can rationalize why molecular. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Describe traits of bonding and antibonding molecular orbitals. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule.
from semesters.in
A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Describe traits of bonding and antibonding molecular orbitals. Using molecular orbital theory, we can rationalize why molecular. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Unlike an atomic orbital, which is centered on a single atom, a.
Introduction to Molecular Orbital Theory semesters.in
What Is A Molecular Orbital Model Using molecular orbital theory, we can rationalize why molecular. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Using molecular orbital theory, we can rationalize why molecular. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Why do some atoms readily form bonds with each other and other atoms don’t? Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions).
From pressbooks.online.ucf.edu
7.5 Hybrid Atomic Orbitals Chemistry Fundamentals What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Molecular orbital (mo) theory. What Is A Molecular Orbital Model.
From www.youtube.com
CHEMISTRY 101 Molecular Orbital Theory YouTube What Is A Molecular Orbital Model Using molecular orbital theory, we can rationalize why molecular. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital (mo) theory describes the behavior of electrons in a molecule. What Is A Molecular Orbital Model.
From www.thoughtco.com
What Is a Molecular Orbital? What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Why do some atoms readily form bonds with. What Is A Molecular Orbital Model.
From chem.libretexts.org
27 Molecular Orbitals with higher Energy Atomic Orbitals (Extra What Is A Molecular Orbital Model Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Describe traits of bonding and antibonding molecular orbitals. Using molecular orbital theory, we can rationalize why molecular. Why. What Is A Molecular Orbital Model.
From chem.libretexts.org
Molecular Orbital Theory Chemistry LibreTexts What Is A Molecular Orbital Model Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Using molecular orbital theory, we can rationalize why molecular. Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular. What Is A Molecular Orbital Model.
From chem.libretexts.org
8.3 Quantum Numbers for Electrons Chemistry LibreTexts What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy.. What Is A Molecular Orbital Model.
From chem.libretexts.org
10.5 Molecular Orbital Theory Chemistry LibreTexts What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Describe traits of bonding and antibonding molecular orbitals. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an. What Is A Molecular Orbital Model.
From www.numerade.com
Select all of the statements that accurately describe the information What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Using molecular orbital theory, we can rationalize why molecular. Why do some atoms readily form bonds with each other and. What Is A Molecular Orbital Model.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed spatial distribution of electrons in a molecule. What Is A Molecular Orbital Model.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts What Is A Molecular Orbital Model Using molecular orbital theory, we can rationalize why molecular. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism. What Is A Molecular Orbital Model.
From www.youtube.com
Understanding Molecular Orbital Theory YouTube What Is A Molecular Orbital Model Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed. What Is A Molecular Orbital Model.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Using molecular orbital theory, we can rationalize why molecular. Why do some atoms readily form bonds with each other and. What Is A Molecular Orbital Model.
From www.myxxgirl.com
Ppt Molecular Orbital Theory Powerpoint Presentation Id My XXX Hot Girl What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital theory (mo theory) provides an explanation. What Is A Molecular Orbital Model.
From gadget.whatisitwellington.com
[9+] Full Color Molecular Orbital Diagram And The Description [+] ANDROID What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Why do some atoms readily form bonds with each other and other atoms don’t? Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts. What Is A Molecular Orbital Model.
From wisc.pb.unizin.org
Day 6 Molecular Orbitals; Lewis Structures Chemistry 109, Fall 2020 What Is A Molecular Orbital Model Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Why do some atoms readily form bonds with each other and other atoms don’t? Describe traits of bonding. What Is A Molecular Orbital Model.
From chem.libretexts.org
10.5 Molecular Orbital Theory Chemistry LibreTexts What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Why do some atoms readily form bonds. What Is A Molecular Orbital Model.
From socratic.org
What is the shape of forbital??? + Example What Is A Molecular Orbital Model Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Using molecular orbital theory, we can rationalize why molecular. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Molecular orbital (mo) theory. What Is A Molecular Orbital Model.
From www.researchgate.net
3 Molecular orbital diagram of NO. Download Scientific Diagram What Is A Molecular Orbital Model Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Unlike an atomic orbital, which is centered on a single atom, a. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Describe traits of bonding and antibonding molecular. What Is A Molecular Orbital Model.
From www.myxxgirl.com
Molecular Orbital Diagram For Co Diagram Media My XXX Hot Girl What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Unlike an atomic orbital, which is centered on a single atom, a. Describe traits of bonding and antibonding molecular orbitals. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Molecular. What Is A Molecular Orbital Model.
From animalia-life.club
No Molecular Orbital Diagram What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes the. What Is A Molecular Orbital Model.
From study.com
Molecular Orbital Theory Tutorial and Diagrams Video & Lesson What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Why do some atoms readily form bonds with each other and other atoms don’t? Using molecular orbital theory, we can rationalize why molecular. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). A molecular orbital is an allowed. What Is A Molecular Orbital Model.
From geometrytipstekhnik.blogspot.com
37+ Molecular Orbital Geometry Image GM What Is A Molecular Orbital Model Why do some atoms readily form bonds with each other and other atoms don’t? Describe traits of bonding and antibonding molecular orbitals. Unlike an atomic orbital, which is centered on a single atom, a. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Using molecular orbital theory, we. What Is A Molecular Orbital Model.
From mungfali.com
9.8 Molecular Orbital Theory Chemistry Libretexts 22B What Is A Molecular Orbital Model Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the. What Is A Molecular Orbital Model.
From socratic.org
What are nonbonding molecular orbitals? + Example What Is A Molecular Orbital Model Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Describe traits of bonding and antibonding molecular. What Is A Molecular Orbital Model.
From classnotes.org.in
Types of Molecular Orbital Formed Chemical Bonding and Molecular What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in. What Is A Molecular Orbital Model.
From chem.libretexts.org
4.9 Molecular Orbitals Chemistry LibreTexts What Is A Molecular Orbital Model A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Using molecular orbital theory, we can. What Is A Molecular Orbital Model.
From blog.naver.com
[🥼일반화학] 2. 원자의 구조와 원소의 주기율 ① 궤도함수와 전자배열 네이버 블로그 What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Why do some atoms readily form bonds. What Is A Molecular Orbital Model.
From www.myxxgirl.com
Molecular Orbital Model For Metals My XXX Hot Girl What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Why do some atoms readily form bonds with each other and other atoms don’t? Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes. What Is A Molecular Orbital Model.
From socratic.org
What are s,p,d,f orbitals? Socratic What Is A Molecular Orbital Model Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Why do some atoms readily form bonds with each other and other atoms don’t? Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Unlike an atomic orbital, which is centered. What Is A Molecular Orbital Model.
From design1systems.com
How Does the Molecular Orbital Diagram of NO+ Impact its Chemical What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Why do some atoms readily form bonds with each other and other atoms don’t? Describe traits of bonding and antibonding molecular orbitals. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen. What Is A Molecular Orbital Model.
From mungfali.com
Molecular Orbitals Diagrams What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Describe traits of bonding and antibonding molecular orbitals. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular. What Is A Molecular Orbital Model.
From www.studocu.com
Activity 10 Molecular Orbitals Activity 10 What Is a Molecular What Is A Molecular Orbital Model Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Describe traits of bonding and antibonding molecular orbitals. Using molecular orbital theory, we can rationalize why molecular. Molecular orbital theory (mo theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Unlike an atomic orbital,. What Is A Molecular Orbital Model.
From www.myxxgirl.com
The Molecular Orbital Diagram Of No Shown In Figure My XXX Hot Girl What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital (mo) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions). Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Describe traits of bonding and antibonding molecular orbitals.. What Is A Molecular Orbital Model.
From semesters.in
Introduction to Molecular Orbital Theory semesters.in What Is A Molecular Orbital Model Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Unlike an atomic orbital, which is centered on a single atom, a. Molecular orbital theory (mo theory) provides. What Is A Molecular Orbital Model.
From chem.libretexts.org
11.5 Molecular Orbital Theory Chemistry LibreTexts What Is A Molecular Orbital Model Unlike an atomic orbital, which is centered on a single atom, a. Why do some atoms readily form bonds with each other and other atoms don’t? Describe traits of bonding and antibonding molecular orbitals. Molecular orbital (mo) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. Molecular orbital (mo) theory describes. What Is A Molecular Orbital Model.