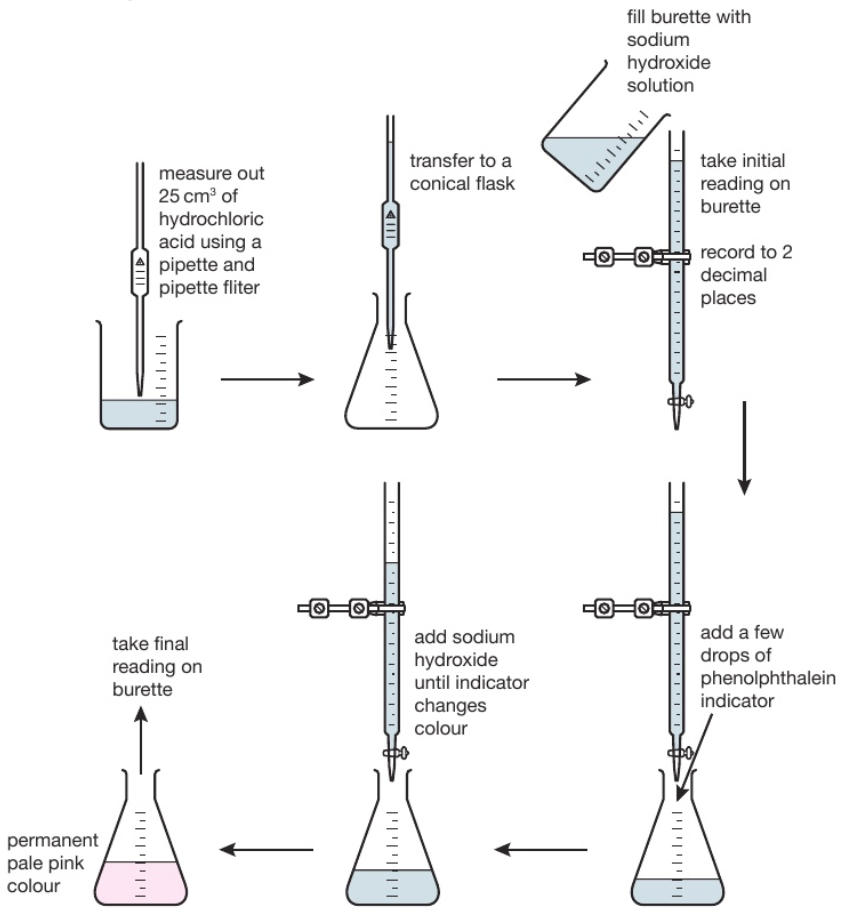
Titration Using Hcl And Naoh . They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. titrate with hcl solution till the first color change. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Repeat titration and boiling till yellow color doesn't return after. In each case the titrant is an.
from www.tutormyself.com
both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. titrate with hcl solution till the first color change. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. In each case the titrant is an. Repeat titration and boiling till yellow color doesn't return after. You have to decide if this experiment is suitable to use with different classes, and look at the need for. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals.
233 (Triple only) describe how to carry out an acidalkali titration
Titration Using Hcl And Naoh in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. titrate with hcl solution till the first color change. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. Repeat titration and boiling till yellow color doesn't return after. In each case the titrant is an. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. You have to decide if this experiment. Titration Using Hcl And Naoh.
From byjus.com
24.Why their is difference when naoh and na2co3 are titrated with Titration Using Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used. Titration Using Hcl And Naoh.
From www.numerade.com
SOLVED 'Determination the normality of a sodium hydroxide solution Titration Using Hcl And Naoh in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In each case the titrant is an. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. both acid and. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. . Titration Using Hcl And Naoh.
From www.solutioninn.com
[Solved] The student performs a second titration u SolutionInn Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Repeat titration and boiling till yellow color doesn't return after. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Titration Using Hcl And Naoh.
From solvedlib.com
A titration of 59.00 ml of NaOH with 0.1000 M HCl was… SolvedLib Titration Using Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. In each case the titrant is an. You have to decide if this experiment is suitable to use with different classes, and look at the need for. titrate with hcl. Titration Using Hcl And Naoh.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Repeat titration and boiling till yellow color doesn't return after. In each case the titrant is an. both acid and. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. titrate with hcl solution till the first color change. They then concentrate the solution and. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. titrate with hcl solution till the first color change. . Titration Using Hcl And Naoh.
From www.studypool.com
SOLUTION Solution for if you used no antacid tablet in your titration Titration Using Hcl And Naoh In each case the titrant is an. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. They then concentrate the. Titration Using Hcl And Naoh.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Titration Using Hcl And Naoh in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In each case the titrant is an. titrate with hcl solution till the first color change. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a titration. Titration Using Hcl And Naoh.
From giomzfpyz.blob.core.windows.net
Titration Of Naoh By H2So4 at Judith Phillips blog Titration Using Hcl And Naoh titrate with hcl solution till the first color change. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Repeat. Titration Using Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. as seen in the chapter. Titration Using Hcl And Naoh.
From www.aiophotoz.com
Difference Between Redox Titration And Acid Base Titration Images Titration Using Hcl And Naoh You have to decide if this experiment is suitable to use with different classes, and look at the need for. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. In each case the titrant is an. a titration is. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh In each case the titrant is an. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Repeat titration and boiling till yellow color doesn't return. Titration Using Hcl And Naoh.
From exysrekix.blob.core.windows.net
AcidBase Titration Lab Report Answers Hcl And Naoh at Van Hart blog Titration Using Hcl And Naoh titrate with hcl solution till the first color change. a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. Repeat titration and boiling till yellow color doesn't return after. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In each case the titrant is an. titrate with hcl solution till the first color change. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride. Titration Using Hcl And Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Using Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. In each case the titrant is an. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a titration is. Titration Using Hcl And Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Using Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. Repeat titration and boiling till yellow color doesn't return after. In each case the titrant is an. as seen in the chapter on the stoichiometry of chemical reactions, titrations can. Titration Using Hcl And Naoh.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Titration Using Hcl And Naoh both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. in this experiment students neutralise sodium. Titration Using Hcl And Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Using Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. You have to decide if this experiment is suitable to use with different classes, and look at the need for. titrate with hcl solution till the first color change. . Titration Using Hcl And Naoh.
From giounmqmo.blob.core.windows.net
Thermometric Titration Of Hcl And Naoh at Barry Montoya blog Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. titrate with hcl solution till. Titration Using Hcl And Naoh.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Repeat. Titration Using Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. titrate with hcl solution till the first color change. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In each case the titrant is an. a titration is carried out for 25.00 ml. Titration Using Hcl And Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. In each case the titrant is an. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. both acid and. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. You have to decide if this experiment is suitable to use with different classes, and look at the need for. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. in this experiment students neutralise. Titration Using Hcl And Naoh.
From www.sliderbase.com
Titration Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. titrate with hcl solution till the first color change. both acid and base are. Titration Using Hcl And Naoh.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Using Hcl And Naoh as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Repeat titration and boiling till yellow color doesn't return after. titrate with hcl solution till the first color change. both acid and base are strong, which not only makes determination of end point easy. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. as seen in the chapter on the stoichiometry of chemical. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh In each case the titrant is an. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Repeat titration and boiling till yellow color doesn't return. Titration Using Hcl And Naoh.
From loehmowzt.blob.core.windows.net
Indicator Used In Titration Of Naoh And Hcl at Dan Carlson blog Titration Using Hcl And Naoh In each case the titrant is an. titrate with hcl solution till the first color change. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Repeat titration and boiling till yellow color doesn't return after. in this experiment students neutralise sodium hydroxide with. Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. as seen in the chapter on the stoichiometry of chemical. Titration Using Hcl And Naoh.
From exyttjuiw.blob.core.windows.net
How To Calculate The Concentration Of Naoh In A Titration at Randy Titration Using Hcl And Naoh a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In each case the titrant is an. Repeat titration and boiling till yellow color doesn't return. Titration Using Hcl And Naoh.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Using Hcl And Naoh Repeat titration and boiling till yellow color doesn't return after. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. both acid and base are strong, which not only makes determination of end point easy (steep part of the curve is long), but also means that calculation of. . Titration Using Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Using Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In each case the titrant is an. You have to decide if this experiment is suitable to use with different classes,. Titration Using Hcl And Naoh.