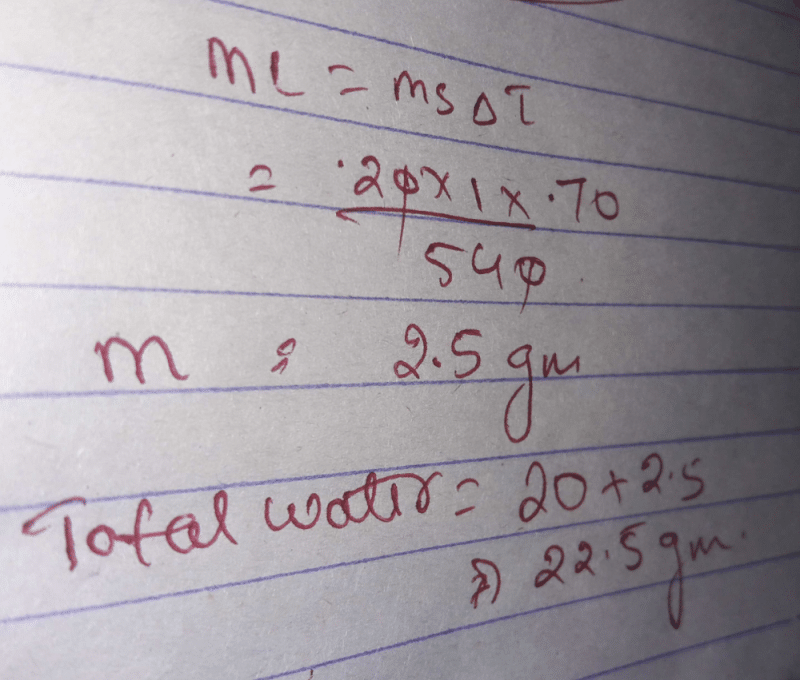What Happens When You Heat Water To 100 Degrees Celsius . At these conditions, you can drill a hole into container and store water. The process due to which a liquid changes. when water is heated to 100°c, boiling of water takes place. If you boil water at a higher pressure (below. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. you can store water at any temperature below 100°c. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). What happens instead is that the water. when heat is added to a pure body of water at 100° celsius the temperature does not change.
from edurev.in
The process due to which a liquid changes. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. you can store water at any temperature below 100°c. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: At these conditions, you can drill a hole into container and store water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. when heat is added to a pure body of water at 100° celsius the temperature does not change. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). If you boil water at a higher pressure (below. when water is heated to 100°c, boiling of water takes place.
Steam at 100 degree celsius is passed into 20 g of water at the 10
What Happens When You Heat Water To 100 Degrees Celsius when water is heated to 100°c, boiling of water takes place. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). when water is heated to 100°c, boiling of water takes place. What happens instead is that the water. when heat is added to a pure body of water at 100° celsius the temperature does not change. The process due to which a liquid changes. If you boil water at a higher pressure (below. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. you can store water at any temperature below 100°c. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. At these conditions, you can drill a hole into container and store water. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature:
From fyoovlsbp.blob.core.windows.net
Hot Water Degrees Celsius at Mary Velasquez blog What Happens When You Heat Water To 100 Degrees Celsius when water is heated to 100°c, boiling of water takes place. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). our water heating calculator can help you determine. What Happens When You Heat Water To 100 Degrees Celsius.
From exyypotyv.blob.core.windows.net
Chicken Breast Cooking Time Oven Celsius at William Barrow blog What Happens When You Heat Water To 100 Degrees Celsius imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when water is heated to 100°c, boiling of water takes place. you can store water at any temperature below 100°c. our water heating calculator can help you determine both the amount of heat required to raise. What Happens When You Heat Water To 100 Degrees Celsius.
From www.pinterest.com
Celsius Scale Easy Science Easy science, Celsius scale, Scale What Happens When You Heat Water To 100 Degrees Celsius At these conditions, you can drill a hole into container and store water. The process due to which a liquid changes. you can store water at any temperature below 100°c. when heat is added to a pure body of water at 100° celsius the temperature does not change. this plot of temperature shows what happens to a. What Happens When You Heat Water To 100 Degrees Celsius.
From www.myxxgirl.com
What Is The Effect Of Temperature On States Of Matter Sciencing My What Happens When You Heat Water To 100 Degrees Celsius At these conditions, you can drill a hole into container and store water. What happens instead is that the water. If you boil water at a higher pressure (below. you can store water at any temperature below 100°c. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature:. What Happens When You Heat Water To 100 Degrees Celsius.
From www.chegg.com
Solved Thermodynamics 1. Determine The Specific Enthalpy What Happens When You Heat Water To 100 Degrees Celsius our water heating calculator can help you determine both the amount of heat required to raise the temperature of. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). . What Happens When You Heat Water To 100 Degrees Celsius.
From giofqzkfj.blob.core.windows.net
Thermometer Temperature Should Be at Pablo Gage blog What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. when water is heated to 100°c, boiling of water takes place. If you boil water at a higher pressure (below. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). you. What Happens When You Heat Water To 100 Degrees Celsius.
From exyksuwcz.blob.core.windows.net
What Is Fridge Temp In Celsius at Sandra Trosper blog What Happens When You Heat Water To 100 Degrees Celsius our water heating calculator can help you determine both the amount of heat required to raise the temperature of. If you boil water at a higher pressure (below. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). imagine a teaspoon of boiling water at 100 degrees celsius (°c) and. What Happens When You Heat Water To 100 Degrees Celsius.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Happens When You Heat Water To 100 Degrees Celsius imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when heat is added to a pure body of water at 100° celsius the temperature does not change. when water is heated to 100°c, boiling of water takes place. The process due to which a liquid changes.. What Happens When You Heat Water To 100 Degrees Celsius.
From learningschoolzazobezx.z22.web.core.windows.net
C To F Conversion Chart Pdf What Happens When You Heat Water To 100 Degrees Celsius this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when water is heated to 100°c, boiling of water. What Happens When You Heat Water To 100 Degrees Celsius.
From exyyyralk.blob.core.windows.net
Can Water Evaporate Below Its Boiling Point at Joanne Ordonez blog What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. At these conditions, you can drill a hole into container and store water. What happens instead is that the water. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). The process due. What Happens When You Heat Water To 100 Degrees Celsius.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? What Happens When You Heat Water To 100 Degrees Celsius at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c. What Happens When You Heat Water To 100 Degrees Celsius.
From studydenudation.z21.web.core.windows.net
What Is 67 Fahrenheit In Celsius What Happens When You Heat Water To 100 Degrees Celsius If you boil water at a higher pressure (below. when heat is added to a pure body of water at 100° celsius the temperature does not change. What happens instead is that the water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added. What Happens When You Heat Water To 100 Degrees Celsius.
From byjus.com
Relation Between Celsius And Fahrenheit at BYJU’S What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when water is heated to 100°c, boiling of water takes place. this plot of temperature shows what happens. What Happens When You Heat Water To 100 Degrees Celsius.
From www.chegg.com
Solved As shown in the figure below, you have a system What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. What happens instead is that the water. The process due to which a liquid changes. at sea level, water boils. What Happens When You Heat Water To 100 Degrees Celsius.
From dilenglish.com
Conditionals What Happens When You Heat Water To 100 Degrees Celsius imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. What happens instead is that the water. At these conditions,. What Happens When You Heat Water To 100 Degrees Celsius.
From www.worldatlas.com
What is the Freezing Point in Celsius? WorldAtlas What Happens When You Heat Water To 100 Degrees Celsius imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: At these conditions, you can drill a hole into container and store water. If you boil water at a higher pressure (below. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32. What Happens When You Heat Water To 100 Degrees Celsius.
From studynonviolent.z13.web.core.windows.net
20 Degrees Celsius To Fahrenheit What Happens When You Heat Water To 100 Degrees Celsius this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. At these conditions, you can drill a hole into container and store water. you can store water at any temperature below 100°c. when water is heated to 100°c, boiling. What Happens When You Heat Water To 100 Degrees Celsius.
From www.thoughtco.com
Difference Between Celsius and Centigrade What Happens When You Heat Water To 100 Degrees Celsius our water heating calculator can help you determine both the amount of heat required to raise the temperature of. when water is heated to 100°c, boiling of water takes place. you can store water at any temperature below 100°c. What happens instead is that the water. when heat is added to a pure body of water. What Happens When You Heat Water To 100 Degrees Celsius.
From www.vectorstock.com
Heat thermometer 40 degrees celsius summer Vector Image What Happens When You Heat Water To 100 Degrees Celsius At these conditions, you can drill a hole into container and store water. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. What happens instead is that the water. you can store water at any temperature below 100°c. when water is heated to 100°c, boiling of water. What Happens When You Heat Water To 100 Degrees Celsius.
From www.vecteezy.com
Boiling water. 100 degree water on pan with thermometer symbol in What Happens When You Heat Water To 100 Degrees Celsius our water heating calculator can help you determine both the amount of heat required to raise the temperature of. The process due to which a liquid changes. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. when heat. What Happens When You Heat Water To 100 Degrees Celsius.
From edurev.in
Steam at 100 degree celsius is passed into 20 g of water at the 10 What Happens When You Heat Water To 100 Degrees Celsius when water is heated to 100°c, boiling of water takes place. If you boil water at a higher pressure (below. What happens instead is that the water. The process due to which a liquid changes. you can store water at any temperature below 100°c. when heat is added to a pure body of water at 100° celsius. What Happens When You Heat Water To 100 Degrees Celsius.
From brainly.in
300gm of water at 25degree celcius is added to 100 gm of ice at 0 What Happens When You Heat Water To 100 Degrees Celsius The process due to which a liquid changes. when heat is added to a pure body of water at 100° celsius the temperature does not change. If you boil water at a higher pressure (below. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: At these conditions,. What Happens When You Heat Water To 100 Degrees Celsius.
From ar.alshueae.net
If you heat water to 100 degrees Celsius, it الشعاع What Happens When You Heat Water To 100 Degrees Celsius At these conditions, you can drill a hole into container and store water. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: If you boil water at a higher pressure (below. What happens instead is that the water. our water heating calculator can help you determine both. What Happens When You Heat Water To 100 Degrees Celsius.
From sciexaminer.com
Scientists heatup water to 100,000 degrees Celsius in a fraction of a What Happens When You Heat Water To 100 Degrees Celsius At these conditions, you can drill a hole into container and store water. What happens instead is that the water. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). . What Happens When You Heat Water To 100 Degrees Celsius.
From www.mprnews.org
100 degree heat index Sunday, late July heat wave MPR News What Happens When You Heat Water To 100 Degrees Celsius this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). What happens instead is that the water. you can store water at. What Happens When You Heat Water To 100 Degrees Celsius.
From fyozkayiz.blob.core.windows.net
Why Does Water Boil At 100 Degrees Celsius At Sea Level at Jesus Newman What Happens When You Heat Water To 100 Degrees Celsius you can store water at any temperature below 100°c. If you boil water at a higher pressure (below. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when water is heated to 100°c, boiling of water takes place. At these conditions, you can drill a hole. What Happens When You Heat Water To 100 Degrees Celsius.
From cedobmcr.blob.core.windows.net
How Hot Are Microwaves at Bruce Hartwig blog What Happens When You Heat Water To 100 Degrees Celsius If you boil water at a higher pressure (below. What happens instead is that the water. The process due to which a liquid changes. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). our water heating calculator can help you determine both the amount of heat required to raise the. What Happens When You Heat Water To 100 Degrees Celsius.
From bmxracingthailand.com
How Hot Is 90 Degrees Celsius? Update What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. At these conditions, you can drill a hole into container and store water. The process due to which a liquid changes. What happens instead is that the water. this plot of temperature shows what happens to a 75 g sample. What Happens When You Heat Water To 100 Degrees Celsius.
From primaryleap.co.uk
Maths Greater Than And Less Than Degrees Celsius Level 1 activity for What Happens When You Heat Water To 100 Degrees Celsius when heat is added to a pure body of water at 100° celsius the temperature does not change. If you boil water at a higher pressure (below. you can store water at any temperature below 100°c. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. What Happens When You Heat Water To 100 Degrees Celsius.
From pixmob.info
Cuanto Es 33 Grados Fahrenheit En Centigrados PIXMOB What Happens When You Heat Water To 100 Degrees Celsius What happens instead is that the water. you can store water at any temperature below 100°c. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. What Happens When You Heat Water To 100 Degrees Celsius.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation What Happens When You Heat Water To 100 Degrees Celsius If you boil water at a higher pressure (below. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. . What Happens When You Heat Water To 100 Degrees Celsius.
From www.meritnation.com
A tap supplies water at 10 degree Celsius and another tab 100 degree What Happens When You Heat Water To 100 Degrees Celsius imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: when heat is added to a pure body of water at 100° celsius the temperature does not change. our water heating calculator can help you determine both the amount of heat required to raise the temperature of.. What Happens When You Heat Water To 100 Degrees Celsius.
From www.tessshebaylo.com
Equation For Heat Energy Change Tessshebaylo What Happens When You Heat Water To 100 Degrees Celsius at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. imagine a teaspoon of boiling water at 100 degrees celsius (°c) and. What Happens When You Heat Water To 100 Degrees Celsius.
From askfilo.com
Water boils at 100 degrees Celsius (212 degrees Fahrenheit). Filo What Happens When You Heat Water To 100 Degrees Celsius What happens instead is that the water. The process due to which a liquid changes. when heat is added to a pure body of water at 100° celsius the temperature does not change. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. you can store water at. What Happens When You Heat Water To 100 Degrees Celsius.
From www.youtube.com
What is 100 degrees Fahrenheit in Celsius ? QnA Explained YouTube What Happens When You Heat Water To 100 Degrees Celsius when water is heated to 100°c, boiling of water takes place. What happens instead is that the water. when heat is added to a pure body of water at 100° celsius the temperature does not change. at sea level, water boils at 100 °c (212 °f) and freezes at 0 °c (32 °f). our water heating. What Happens When You Heat Water To 100 Degrees Celsius.