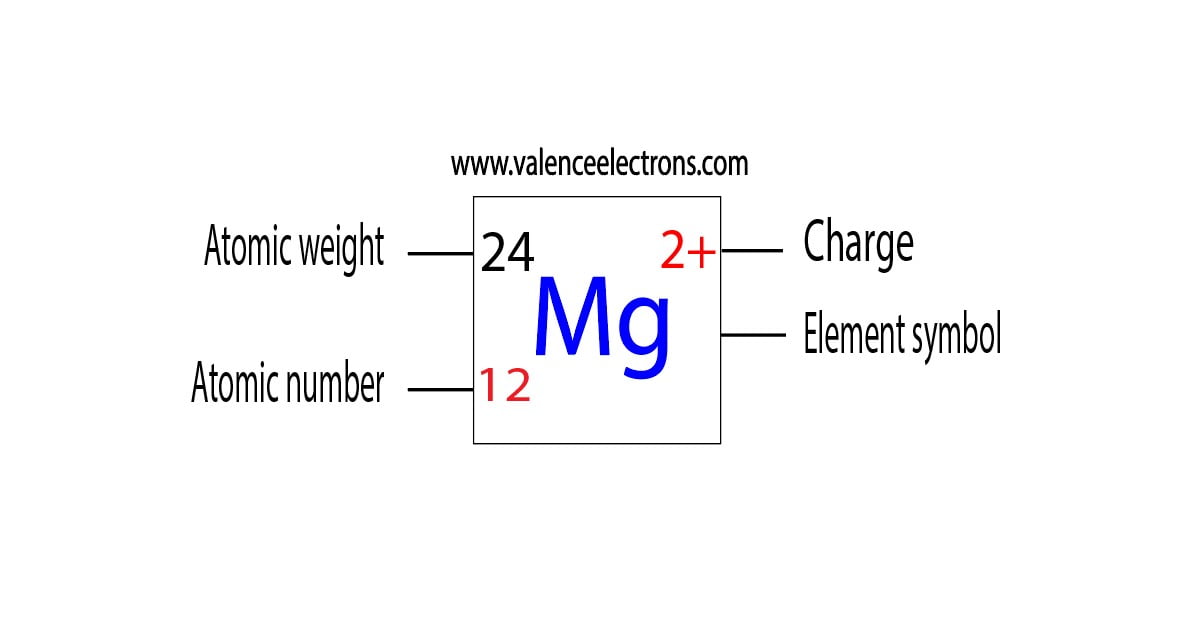
Magnesium Charge Of Ion . 93 rows ionic charge: Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This electric charge generated on the ion is. Magnesium atoms contain 12 protons in their nucleus. The symbol for the ion is mg 2+ , and it is called a. To get a +2 charge, the ion had to lose two electrons. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: A magnesium ion therefore has. Writing formulae of ionic compounds. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Metals form positive ions (cations). Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
from valenceelectrons.com
When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Magnesium atoms contain 12 protons in their nucleus. To get a +2 charge, the ion had to lose two electrons. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. This electric charge generated on the ion is. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Writing formulae of ionic compounds. Metals form positive ions (cations). \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges.
How to Write the Electron Configuration for Magnesium (Mg)?
Magnesium Charge Of Ion This electric charge generated on the ion is. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Writing formulae of ionic compounds. A magnesium ion therefore has. 93 rows ionic charge: To get a +2 charge, the ion had to lose two electrons. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Metals form positive ions (cations). Magnesium atoms contain 12 protons in their nucleus. The symbol for the ion is mg 2+ , and it is called a. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge Of Ion A magnesium ion therefore has. 93 rows ionic charge: Metals form positive ions (cations). When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Writing formulae of ionic compounds. This electric charge generated on the ion is. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and. Magnesium Charge Of Ion.
From www.researchgate.net
Schematic illustrations of the behavior of the magnesium ions at the Magnesium Charge Of Ion \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Magnesium atoms contain 12 protons in their. Magnesium Charge Of Ion.
From www.youtube.com
magnesium ion charge YouTube Magnesium Charge Of Ion This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Metals form positive ions (cations). Writing formulae of ionic compounds. To get a +2 charge, the ion had to lose two electrons. 93 rows ionic charge: Magnesium atoms contain 12 protons in. Magnesium Charge Of Ion.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Charge Of Ion Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: This electric charge generated on the ion is. A magnesium atom must. Magnesium Charge Of Ion.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Charge Of Ion A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: To get a +2 charge, the ion had to lose two electrons. This electric charge generated on the ion is. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Learn about and revise equations and. Magnesium Charge Of Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Charge Of Ion Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. To get a +2 charge, the ion. Magnesium Charge Of Ion.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Charge Of Ion A magnesium ion therefore has. Writing formulae of ionic compounds. 93 rows ionic charge: This electric charge generated on the ion is. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. Magnesium Charge Of Ion.
From brainly.com
What is the charge on a magnesium ion? How do they get that charge Magnesium Charge Of Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium atoms contain 12 protons in their nucleus. 93 rows ionic charge: Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance. Magnesium Charge Of Ion.
From www.showme.com
Magnesium + Oxygen Ionic Bonding Science, Chemistry ShowMe Magnesium Charge Of Ion This electric charge generated on the ion is. Metals form positive ions (cations). Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. 93 rows ionic charge: A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Magnesium atoms contain 12 protons in their nucleus. Writing. Magnesium Charge Of Ion.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Charge Of Ion Magnesium atoms contain 12 protons in their nucleus. Writing formulae of ionic compounds. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: This electric charge generated on the ion is. A magnesium ion therefore has. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Learn about and revise. Magnesium Charge Of Ion.
From www.slideshare.net
Bonding Basics Magnesium Charge Of Ion Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. To get a +2 charge, the ion had to lose two electrons. Metals form positive ions (cations). This electric charge generated on the ion is. Writing formulae of ionic compounds. A magnesium ion therefore has. Magnesium atoms contain 12 protons in their. Magnesium Charge Of Ion.
From www.alamy.com
Symbol and electron diagram for Magnesium illustration Stock Vector Magnesium Charge Of Ion Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This electric charge generated on the ion is. To get a +2 charge, the ion had to lose two electrons. 93 rows ionic charge: Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study. Magnesium Charge Of Ion.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Charge Of Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). To get a +2 charge, the ion had to lose two electrons. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Thus, a magnesium atom will form a cation with two fewer electrons than. Magnesium Charge Of Ion.
From www.slideshare.net
The periodic table Magnesium Charge Of Ion A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Writing formulae of ionic compounds. To get a +2 charge, the ion had to lose two electrons. Magnesium atoms contain 12 protons in their nucleus. A magnesium. Magnesium Charge Of Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Charge Of Ion 93 rows ionic charge: Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. To get a +2 charge, the ion had to lose two electrons. The symbol for the ion is mg 2+ , and it is called a. Writing formulae of ionic compounds. Thus, a magnesium atom will form a. Magnesium Charge Of Ion.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Charge Of Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Learn about and revise equations and. Magnesium Charge Of Ion.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Charge Of Ion Metals form positive ions (cations). This electric charge generated on the ion is. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. The symbol for the ion. Magnesium Charge Of Ion.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Charge Of Ion This electric charge generated on the ion is. A magnesium ion therefore has. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Writing formulae of ionic compounds. The symbol for the ion is mg 2+ , and it is called a. To get a +2 charge, the ion had to. Magnesium Charge Of Ion.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium Charge Of Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. Writing formulae of ionic compounds. 93 rows ionic charge: Magnesium atoms contain 12 protons in their nucleus. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: This electric charge generated on the ion is. A. Magnesium Charge Of Ion.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Charge Of Ion \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. The symbol for the ion is mg 2+ , and it is called a. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: A magnesium atom must lose two electrons to have the same number electrons as an atom. Magnesium Charge Of Ion.
From spmkimia.onlinetuition.com.my
Pembentukan Ion Positif Nota Ulangkaji Kimia SPM Tingkatan 4/Tingkatan 5 Magnesium Charge Of Ion 93 rows ionic charge: This electric charge generated on the ion is. The symbol for the ion is mg 2+ , and it is called a. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry. Magnesium Charge Of Ion.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Magnesium Oxide MgO Stock Illustration Magnesium Charge Of Ion Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+ , and it is called a. Metals form positive ions (cations). \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Writing formulae of ionic compounds. When the. Magnesium Charge Of Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Charge Of Ion Magnesium atoms contain 12 protons in their nucleus. 93 rows ionic charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. This electric charge generated on the ion is. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: When the atom loses or gains one or more electrons,. Magnesium Charge Of Ion.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Charge Of Ion Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. Magnesium atoms contain 12 protons in their nucleus. A magnesium ion therefore has. The symbol for the ion is mg 2+ , and it is called a. Writing formulae of ionic compounds. 93 rows ionic charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each. Magnesium Charge Of Ion.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Charge Of Ion To get a +2 charge, the ion had to lose two electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium atoms contain 12 protons in their nucleus. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: A magnesium ion therefore has.. Magnesium Charge Of Ion.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Charge Of Ion Magnesium atoms contain 12 protons in their nucleus. Metals form positive ions (cations). A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. A magnesium ion therefore has. A magnesium atom must lose two electrons to have the same number. Magnesium Charge Of Ion.
From mavink.com
Draw The Orbital Diagram For Magnesium Magnesium Charge Of Ion Magnesium atoms contain 12 protons in their nucleus. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. 93 rows ionic charge: To get a +2 charge, the. Magnesium Charge Of Ion.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Charge Of Ion To get a +2 charge, the ion had to lose two electrons. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. The symbol for the ion is mg 2+ , and it is called a. Metals. Magnesium Charge Of Ion.
From slideplayer.com
Chemical Bonds. ppt download Magnesium Charge Of Ion Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium ion therefore has. 93 rows ionic charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. Metals form positive ions (cations). When the atom loses or gains one or more electrons, the electric. Magnesium Charge Of Ion.
From www.slideserve.com
PPT Quiz Review PowerPoint Presentation, free download ID2732026 Magnesium Charge Of Ion The symbol for the ion is mg 2+ , and it is called a. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. To get a. Magnesium Charge Of Ion.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Charge Of Ion Metals form positive ions (cations). Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Writing formulae of ionic compounds. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges. The. Magnesium Charge Of Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Magnesium Charge Of Ion This electric charge generated on the ion is. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous. To get a +2 charge, the ion had to lose two electrons. The symbol for the ion is mg 2+ , and it is called a. Magnesium atoms. Magnesium Charge Of Ion.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Charge Of Ion The symbol for the ion is mg 2+ , and it is called a. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this bbc bitesize gcse chemistry (ocr 21c) study guide. This electric charge generated on the ion is. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Magnesium Charge Of Ion.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the Magnesium Charge Of Ion A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Magnesium atoms contain 12 protons in their nucleus. The symbol for the ion is mg 2+ , and it is called a. This electric charge generated on the ion is. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely balance the positive and negative charges.. Magnesium Charge Of Ion.
From www.chegg.com
Solved Magnesium form ions with a charge of? a. 0 b. 2− c. Magnesium Charge Of Ion This electric charge generated on the ion is. Magnesium atoms contain 12 protons in their nucleus. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The symbol for the ion is mg 2+ , and it is called a. Writing formulae of ionic compounds. Learn about and revise equations and chemical reactions with this. Magnesium Charge Of Ion.