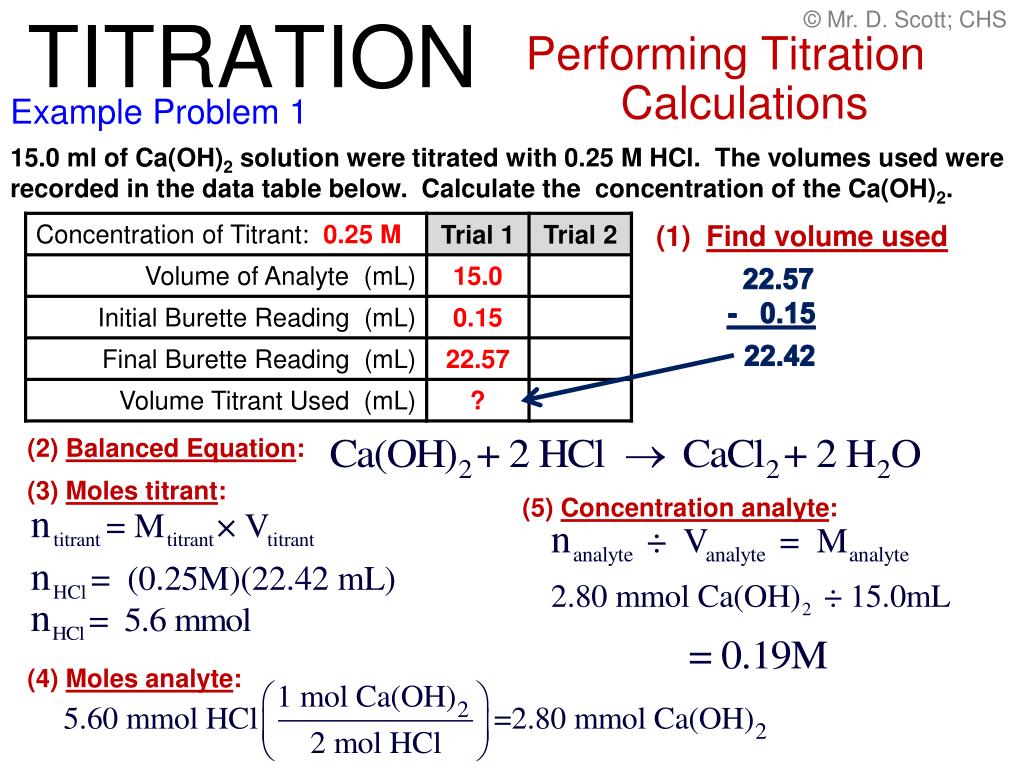
At The Equivalence Point Of A Titration What Is Equal . For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. At this specific point, the. that particular mixture is known as the equivalence point. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
from hxesplqrv.blob.core.windows.net
they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. that particular mixture is known as the equivalence point. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At this specific point, the.
Titration Balanced Equation Formula at Brandon Palmer blog
At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. that particular mixture is known as the equivalence point.
From www.chegg.com
Solved QUESTION 4In an acidbase titration, the moles of the At The Equivalence Point Of A Titration What Is Equal — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to. At The Equivalence Point Of A Titration What Is Equal.
From www.youtube.com
Titration Curves, Equivalence Point YouTube At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to. At The Equivalence Point Of A Titration What Is Equal.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. For example, if. At The Equivalence Point Of A Titration What Is Equal.
From educationsupply.z13.web.core.windows.net
How To Determine Equivalence Point At The Equivalence Point Of A Titration What Is Equal For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. a point of equivalence in a titration. At The Equivalence Point Of A Titration What Is Equal.
From dxozbvbve.blob.core.windows.net
Titration Weak Acid Weak Base at Minnie Cortez blog At The Equivalence Point Of A Titration What Is Equal they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. At the equivalence point in a neutralization, the moles of acid are equal to the moles. At The Equivalence Point Of A Titration What Is Equal.
From goodttorials.blogspot.com
How To Find Equivalence Point Titration At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. a point of equivalence in a titration refers to a point at which the added. At The Equivalence Point Of A Titration What Is Equal.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. that particular mixture is known as the equivalence point. For example, if you were titrating sodium hydroxide solution with hydrochloric. At The Equivalence Point Of A Titration What Is Equal.
From www.youtube.com
How to Calculate the Volume of Titrant Needed to Reach Equivalence At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. that particular mixture is known. At The Equivalence Point Of A Titration What Is Equal.
From hxeixtrjs.blob.core.windows.net
Difference Between Halfway Point And Equivalence Point at Jennifer Hay blog At The Equivalence Point Of A Titration What Is Equal — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. they look for an “equivalence. At The Equivalence Point Of A Titration What Is Equal.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. a point of equivalence in a titration refers to a. At The Equivalence Point Of A Titration What Is Equal.
From www.aiophotoz.com
How To Find The Half Equivalence Point In A Titration Graph Sciencing At The Equivalence Point Of A Titration What Is Equal we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a point of equivalence in a titration refers to a. At The Equivalence Point Of A Titration What Is Equal.
From hxeppfxlk.blob.core.windows.net
Titration Equivalence Point Endpoint at Nicole Dubois blog At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. For example, if you were titrating sodium hydroxide solution. At The Equivalence Point Of A Titration What Is Equal.
From dxonqncbl.blob.core.windows.net
Titration Problems Weak Acid Strong Base at Joan Tijerina blog At The Equivalence Point Of A Titration What Is Equal At this specific point, the. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At the equivalence. At The Equivalence Point Of A Titration What Is Equal.
From schoolbag.info
Titration and Buffers Acids and Bases At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. At the equivalence point in a neutralization, the moles. At The Equivalence Point Of A Titration What Is Equal.
From hxehwxley.blob.core.windows.net
Volume Titration Equivalence Point at Shirley Brothers blog At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At this specific point, the. . At The Equivalence Point Of A Titration What Is Equal.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. At this specific point, the. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. they look for an “equivalence point,” the point. At The Equivalence Point Of A Titration What Is Equal.
From www.chegg.com
Solved The titration curve below shows the results of the At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. At this specific point, the. they look for an “equivalence point,” the point at which enough titrant has. At The Equivalence Point Of A Titration What Is Equal.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First At The Equivalence Point Of A Titration What Is Equal they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. At this specific point, the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — the equivalence point of a chemical reaction is the point at which equal quantities. At The Equivalence Point Of A Titration What Is Equal.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie At The Equivalence Point Of A Titration What Is Equal they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. For example, if. At The Equivalence Point Of A Titration What Is Equal.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co At The Equivalence Point Of A Titration What Is Equal we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. that particular mixture is known as the equivalence point. At. At The Equivalence Point Of A Titration What Is Equal.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube At The Equivalence Point Of A Titration What Is Equal we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. a point. At The Equivalence Point Of A Titration What Is Equal.
From exojtabwh.blob.core.windows.net
Titration Diprotic Acid at Amy Long blog At The Equivalence Point Of A Titration What Is Equal a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. that particular mixture is known as the equivalence point. At this specific point, the. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. — the equivalence. At The Equivalence Point Of A Titration What Is Equal.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. — the equivalence point of. At The Equivalence Point Of A Titration What Is Equal.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. . At The Equivalence Point Of A Titration What Is Equal.
From hxeptiewa.blob.core.windows.net
Find Equivalence Point From Titration at Roger Cornelius blog At The Equivalence Point Of A Titration What Is Equal we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. At this specific point, the. that particular mixture is known as the equivalence point. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. At the equivalence point in a neutralization, the moles. At The Equivalence Point Of A Titration What Is Equal.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog At The Equivalence Point Of A Titration What Is Equal For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. we begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 ml. they look for an “equivalence. At The Equivalence Point Of A Titration What Is Equal.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators At The Equivalence Point Of A Titration What Is Equal At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. that particular mixture is known as the equivalence point. we begin by calculating the titration’s equivalence point. At The Equivalence Point Of A Titration What Is Equal.
From hxesplqrv.blob.core.windows.net
Titration Balanced Equation Formula at Brandon Palmer blog At The Equivalence Point Of A Titration What Is Equal — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. a point of equivalence in a titration. At The Equivalence Point Of A Titration What Is Equal.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point At The Equivalence Point Of A Titration What Is Equal At this specific point, the. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. At the equivalence point in a neutralization, the moles of acid are equal to. At The Equivalence Point Of A Titration What Is Equal.
From hxewhqlyt.blob.core.windows.net
Find The Half Equivalence Point On Titration Curve at Glenda Hopper blog At The Equivalence Point Of A Titration What Is Equal For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. At this specific. At The Equivalence Point Of A Titration What Is Equal.
From ar.inspiredpencil.com
Titration Diagram At The Equivalence Point Of A Titration What Is Equal that particular mixture is known as the equivalence point. For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. At this specific point, the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — the equivalence point of a chemical reaction is the point at which. At The Equivalence Point Of A Titration What Is Equal.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog At The Equivalence Point Of A Titration What Is Equal For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. that particular mixture is known as the equivalence point. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to. At The Equivalence Point Of A Titration What Is Equal.
From uhighlsu.web.fc2.com
Help At The Equivalence Point Of A Titration What Is Equal they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. — the equivalence point of a chemical reaction is the point at which equal quantities of reactants. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. we begin. At The Equivalence Point Of A Titration What Is Equal.
From hxenruftb.blob.core.windows.net
What's The Difference Between An Endpoint And Equivalence Point at At The Equivalence Point Of A Titration What Is Equal a point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. — the equivalence point of a chemical reaction is the point at which. At The Equivalence Point Of A Titration What Is Equal.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder At The Equivalence Point Of A Titration What Is Equal For example, if you were titrating sodium hydroxide solution with hydrochloric acid, both. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At this specific point, the. they look for an “equivalence point,” the point at which enough titrant has combined with the analyte to neutralize it. we begin. At The Equivalence Point Of A Titration What Is Equal.