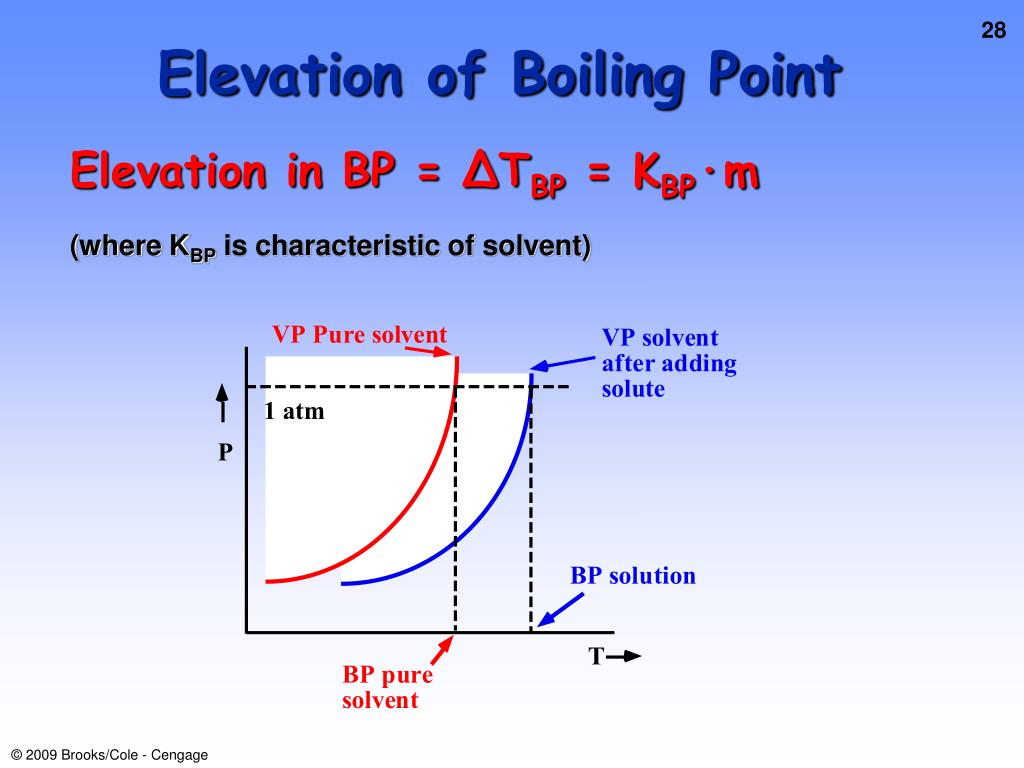
What Is A Boiling Point Elevation . The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). An equation has been developed for. The more solute dissolved, the greater the effect. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. This is the colligative property called boiling point elevation. For example, dissolving salt in water raises the boiling. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of.
from www.slideserve.com
Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. For example, dissolving salt in water raises the boiling. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The boiling points of solutions are all higher than that of the pure solvent. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. This is the colligative property called boiling point elevation. An equation has been developed for. The more solute dissolved, the greater the effect. Difference between the boiling points.
PPT Chapter 14 Solutions and Their Behavior PowerPoint Presentation
What Is A Boiling Point Elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The more solute dissolved, the greater the effect. This is the colligative property called boiling point elevation. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The boiling points of solutions are all higher than that of the pure solvent. An equation has been developed for. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Difference between the boiling points. For example, dissolving salt in water raises the boiling. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Boiling Point Elevation The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). For example, dissolving salt in water raises the boiling. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Solutions PowerPoint Presentation ID2965441 What Is A Boiling Point Elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The more solute dissolved, the greater the effect. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the. What Is A Boiling Point Elevation.
From ar.inspiredpencil.com
Boiling Point Elevation Equation What Is A Boiling Point Elevation This is the colligative property called boiling point elevation. The boiling points of solutions are all higher than that of the pure solvent. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. For example, dissolving salt in water raises the boiling. The boiling point of the solvent above a solution changes. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Chapter 14 Solutions and Their Behavior PowerPoint Presentation What Is A Boiling Point Elevation Difference between the boiling points. An equation has been developed for. This is the colligative property called boiling point elevation. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. For example, dissolving salt in water raises the boiling. The boiling points of solutions are all higher than that. What Is A Boiling Point Elevation.
From inspiritvr.com
Boiling Point Elevation and Freezing Point Depression Study Guide What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. This is the colligative property called boiling point elevation. An equation has been developed for. The boiling points of solutions are all higher than that of the pure solvent. The boiling point of the solvent above a solution changes as the concentration. What Is A Boiling Point Elevation.
From byjus.com
What is elevation in boiling point and depression in freezing point? What Is A Boiling Point Elevation The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). The boiling points of solutions are all higher than that of the pure solvent. Boiling point elevation occurs when the boiling point of a solution. What Is A Boiling Point Elevation.
From www.youtube.com
Boiling Point Elevation with Examples! YouTube What Is A Boiling Point Elevation An equation has been developed for. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. This is the colligative property called boiling point elevation. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not. What Is A Boiling Point Elevation.
From www.youtube.com
Explain Elevation in Boiling Point and Derive equation for molal What Is A Boiling Point Elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. For example, dissolving salt in water raises the boiling. An equation has been developed for. This is the colligative property called boiling point elevation. Boiling point elevation is the increase in the boiling point of a solvent by dissolving. What Is A Boiling Point Elevation.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. The more solute dissolved, the greater the effect. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the. What Is A Boiling Point Elevation.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Boiling Point Elevation Difference between the boiling points. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. An equation has been developed for. The boiling points of solutions are all higher than that of the pure solvent. This is the colligative property called boiling point elevation. Boiling point elevation is the increase in the. What Is A Boiling Point Elevation.
From www.jove.com
11369.jpg What Is A Boiling Point Elevation The boiling points of solutions are all higher than that of the pure solvent. For example, dissolving salt in water raises the boiling. An equation has been developed for. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. The more solute dissolved, the greater the effect. This is the. What Is A Boiling Point Elevation.
From www.youtube.com
Calculating boiling point elevation YouTube What Is A Boiling Point Elevation The boiling points of solutions are all higher than that of the pure solvent. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). For example, dissolving salt in water raises the boiling. Difference between. What Is A Boiling Point Elevation.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. This is the colligative property called boiling point elevation. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or. What Is A Boiling Point Elevation.
From www.pinterest.co.uk
Boiling Point Elevation Easy Science Easy science, Boiling point What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. This is the colligative property called boiling point elevation. The boiling points of solutions are all higher than that of the pure solvent. Difference between the boiling points. For example, dissolving salt in water raises the boiling. Boiling point elevation is the difference in temperature between the boiling point of the pure. What Is A Boiling Point Elevation.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is A Boiling Point Elevation The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). For example, dissolving salt in water raises the boiling. Difference between the boiling points. Boiling point elevation occurs when the boiling point of a solution. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation ID442338 What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. For example, dissolving salt in water raises the boiling. An equation has been developed for. The more solute dissolved, the greater the effect. Difference between the boiling points. This is the colligative property called boiling point elevation. The boiling point of the. What Is A Boiling Point Elevation.
From www.youtube.com
Elevation in boiling point colligative properties Ebullioscopic What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Difference between the boiling points. The more solute dissolved, the greater the effect. The boiling points of solutions are all higher than. What Is A Boiling Point Elevation.
From www.youtube.com
Boiling Point Elevation and Freezing Point Depression YouTube What Is A Boiling Point Elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). This is the colligative. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID37453 What Is A Boiling Point Elevation For example, dissolving salt in water raises the boiling. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Boiling point elevation is the increase in the boiling point of a solvent by dissolving a. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Entry Task Dec 3 rd Monday PowerPoint Presentation, free What Is A Boiling Point Elevation This is the colligative property called boiling point elevation. The more solute dissolved, the greater the effect. An equation has been developed for. Difference between the boiling points. The boiling points of solutions are all higher than that of the pure solvent. For example, dissolving salt in water raises the boiling. The boiling point of the solvent above a solution. What Is A Boiling Point Elevation.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is A Boiling Point Elevation Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The boiling points of solutions are all higher than that of the pure solvent. An equation has been developed for. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID What Is A Boiling Point Elevation Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. For example, dissolving salt in water raises the boiling. This is the colligative property called boiling point elevation. The more solute dissolved, the greater the effect. The boiling point of the solvent above a solution changes as the concentration of the solute. What Is A Boiling Point Elevation.
From www.youtube.com
Boiling Point Elevation/Raoult's Law YouTube What Is A Boiling Point Elevation For example, dissolving salt in water raises the boiling. An equation has been developed for. The more solute dissolved, the greater the effect. The boiling points of solutions are all higher than that of the pure solvent. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does. What Is A Boiling Point Elevation.
From www.youtube.com
Boiling Point Elevation Example YouTube What Is A Boiling Point Elevation The boiling points of solutions are all higher than that of the pure solvent. An equation has been developed for. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. For example, dissolving salt in water raises the boiling. Boiling point elevation is the difference in temperature between the boiling point of. What Is A Boiling Point Elevation.
From www.youtube.com
Derive Equation for Boiling Point Elevation YouTube What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the. What Is A Boiling Point Elevation.
From www.youtube.com
What is Boiling Point Elevation? YouTube What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. This is the colligative property called boiling point elevation. Difference between the boiling. What Is A Boiling Point Elevation.
From www.studocu.com
Boiling Point Elevation Boiling Point Elevation What is the What Is A Boiling Point Elevation This is the colligative property called boiling point elevation. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. Difference between the boiling points. The more solute dissolved, the greater the effect. For example, dissolving salt in water raises the boiling. An equation has been developed for. Boiling point. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Solutions part 2 PowerPoint Presentation, free download ID706322 What Is A Boiling Point Elevation The boiling points of solutions are all higher than that of the pure solvent. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). The more solute dissolved, the greater the effect. For example, dissolving. What Is A Boiling Point Elevation.
From www.fity.club
Boiling Point What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Boiling point elevation is the difference in temperature between the boiling point of the. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Solutions part 2 PowerPoint Presentation ID706322 What Is A Boiling Point Elevation This is the colligative property called boiling point elevation. An equation has been developed for. Boiling point elevation is the difference in temperature between the boiling point of the pure solvent and that of the solution. The more solute dissolved, the greater the effect. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID478383 What Is A Boiling Point Elevation This is the colligative property called boiling point elevation. For example, dissolving salt in water raises the boiling. The more solute dissolved, the greater the effect. The boiling points of solutions are all higher than that of the pure solvent. Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it.. What Is A Boiling Point Elevation.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Boiling Point Elevation Boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Difference between the boiling points. For example, dissolving salt in water raises the boiling. The boiling points of solutions are all higher than that of the pure solvent. Boiling point elevation occurs when the boiling point of a solution becomes. What Is A Boiling Point Elevation.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. This is the colligative property called boiling point elevation. An equation has been developed for. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). The boiling. What Is A Boiling Point Elevation.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point What Is A Boiling Point Elevation The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). The more solute dissolved, the greater the effect. This is the colligative property called boiling point elevation. Boiling point elevation is the difference in temperature. What Is A Boiling Point Elevation.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is A Boiling Point Elevation The more solute dissolved, the greater the effect. The boiling point of the solvent above a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Difference between the boiling points. Boiling point elevation is the difference in temperature between the boiling point. What Is A Boiling Point Elevation.