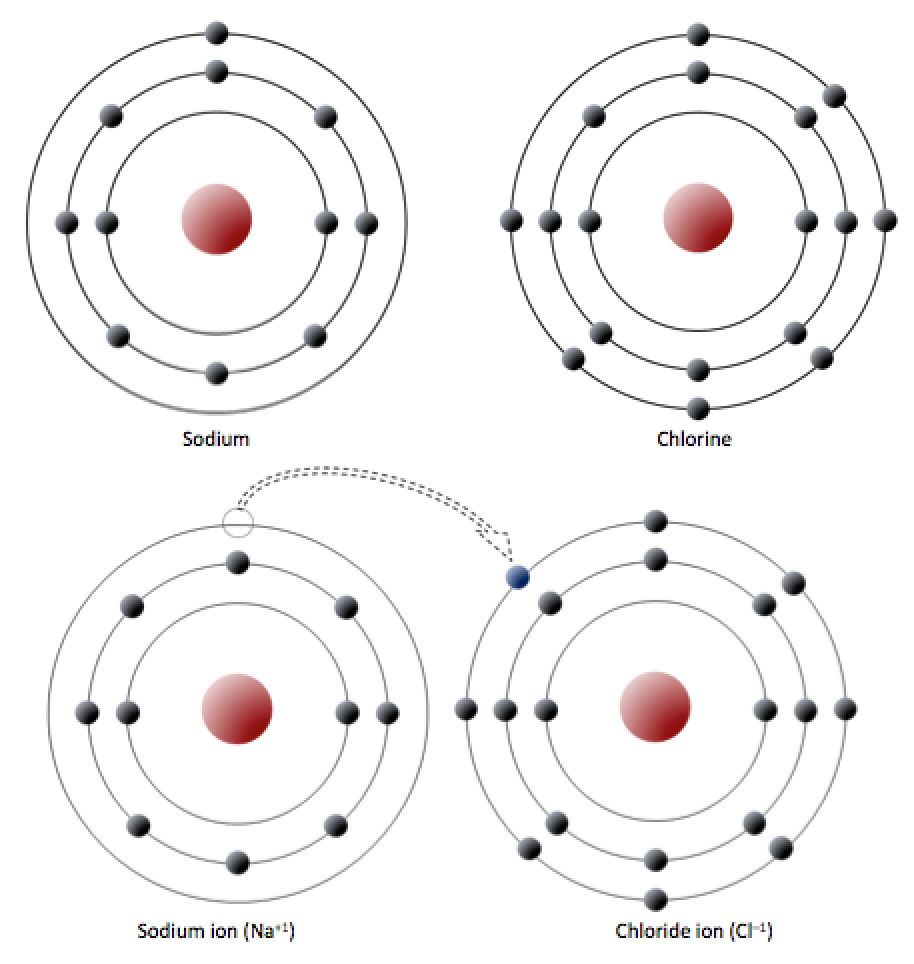
Chlorine Plus Electron . a neutral chlorine atom has seven electrons in its outermost shell. Instead, it now has 17 protons and 18 electrons. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. It has 17 protons in the nucleus. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. We'll need to know how. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). Only one more electron is needed to achieve an octet in.
from opentextbc.ca
a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. Instead, it now has 17 protons and 18 electrons. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). We'll need to know how. Only one more electron is needed to achieve an octet in. a neutral chlorine atom has seven electrons in its outermost shell. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1.
2.2 Bonding and Lattices Physical Geology
Chlorine Plus Electron a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Instead, it now has 17 protons and 18 electrons. We'll need to know how. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. a neutral chlorine atom has seven electrons in its outermost shell. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. It has 17 protons in the nucleus. Only one more electron is needed to achieve an octet in.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Plus Electron small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). Instead, it now has 17 protons and 18 electrons. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. a neutral chlorine atom has seven electrons in its outermost shell. in order. Chlorine Plus Electron.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Plus Electron revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. when a chlorine atom gains an electron, it no longer has the same number of. Chlorine Plus Electron.
From www.dreamstime.com
Atom of Chlorine with Detailed Core and 17 Electrons on White with Chlorine Plus Electron It has 17 protons in the nucleus. a neutral chlorine atom has seven electrons in its outermost shell. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom. Chlorine Plus Electron.
From egpat.com
Lewis dot structure How to write? Chlorine Plus Electron revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Only one more electron is needed to achieve an octet in. by contrast, chlorine. Chlorine Plus Electron.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology Chlorine Plus Electron We'll need to know how. Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. by contrast, chlorine has the electronic structure 1s. Chlorine Plus Electron.
From sciencenotes.org
Chlorine Facts Chlorine Plus Electron Only one more electron is needed to achieve an octet in. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. a neutral chlorine atom has seven electrons in its outermost shell.. Chlorine Plus Electron.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Plus Electron a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. Only one more electron is needed to achieve an octet in. in order to write the chlorine electron configuration we first need to know the. Chlorine Plus Electron.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Plus Electron a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. chlorine has an atomic number of 17,. Chlorine Plus Electron.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Plus Electron It has 17 protons in the nucleus. Only one more electron is needed to achieve an octet in. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. in order to write the chlorine electron configuration we first need to know the number of electrons for the. Chlorine Plus Electron.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Plus Electron Only one more electron is needed to achieve an octet in. Instead, it now has 17 protons and 18 electrons. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. We'll need to know how. revision notes on the reactions of chlorine for the cie a level. Chlorine Plus Electron.
From brainly.in
Draw the electron dot structure of chlorine molecule Brainly.in Chlorine Plus Electron in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). a neutral chlorine atom has seven electrons in its outermost shell. It has 17 protons in the nucleus. Only one more electron. Chlorine Plus Electron.
From www.shutterstock.com
835 en la categoría «Chlorine electrons» de fotos e imágenes de stock Chlorine Plus Electron Only one more electron is needed to achieve an octet in. It has 17 protons in the nucleus. Instead, it now has 17 protons and 18 electrons. a neutral chlorine atom has seven electrons in its outermost shell. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. in. Chlorine Plus Electron.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Plus Electron a neutral chlorine atom has seven electrons in its outermost shell. It has 17 protons in the nucleus. Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). in order to write the chlorine electron configuration we first need to know the number of. Chlorine Plus Electron.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Plus Electron in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save.. Chlorine Plus Electron.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Plus Electron It has 17 protons in the nucleus. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Instead, it now has 17 protons and 18 electrons. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1.. Chlorine Plus Electron.
From www.go4prep.com
Chlorine Electron configuration, Atomic number, Mass, Uses Chlorine Plus Electron It has 17 protons in the nucleus. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. revision notes on the reactions of chlorine for the cie a. Chlorine Plus Electron.
From 2012books.lardbucket.org
Ions Chlorine Plus Electron Instead, it now has 17 protons and 18 electrons. We'll need to know how. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. Only one more electron is. Chlorine Plus Electron.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Plus Electron a neutral chlorine atom has seven electrons in its outermost shell. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Instead, it now has 17 protons and 18 electrons. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. revision notes on the. Chlorine Plus Electron.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Plus Electron Instead, it now has 17 protons and 18 electrons. It has 17 protons in the nucleus. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. in order to write the chlorine electron configuration we. Chlorine Plus Electron.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Plus Electron a neutral chlorine atom has seven electrons in its outermost shell. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It has 17 protons in the nucleus. Instead, it now has 17 protons and 18 electrons. in order to write the chlorine electron configuration we. Chlorine Plus Electron.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Plus Electron by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. It has 17 protons in the nucleus. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Only one more electron is needed to achieve. Chlorine Plus Electron.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine Plus Electron by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. It has 17 protons in the nucleus. a neutral chlorine atom has seven electrons. Chlorine Plus Electron.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Plus Electron Instead, it now has 17 protons and 18 electrons. We'll need to know how. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). Only one more electron is needed to achieve an octet. Chlorine Plus Electron.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Plus Electron a neutral chlorine atom has seven electrons in its outermost shell. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. We'll need to know how. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p. Chlorine Plus Electron.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Plus Electron We'll need to know how. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Only one more electron is needed to achieve an octet in.. Chlorine Plus Electron.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Plus Electron a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Instead, it now has 17 protons and 18 electrons. It has 17 protons in the nucleus. We'll need to know how. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. revision. Chlorine Plus Electron.
From pnghero.com
Lewis Structure Electron Chlorine Diagram Chloride PNG Image PNGHERO Chlorine Plus Electron small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). Instead, it now has 17 protons and 18 electrons. Only one more electron is needed to achieve an octet in. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. revision notes on. Chlorine Plus Electron.
From gardenandplate.com
Molecules Chlorine Plus Electron We'll need to know how. Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). chlorine has an atomic number of 17, which means it has 17 protons and therefore 17. Chlorine Plus Electron.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Plus Electron by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. It has 17 protons in the nucleus. We'll need to know how. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\). a neutral chlorine atom has seven electrons in its outermost shell.. Chlorine Plus Electron.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Plus Electron We'll need to know how. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the. Chlorine Plus Electron.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Plus Electron It has 17 protons in the nucleus. by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. a chlorine atom always gains one electron when. Chlorine Plus Electron.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Plus Electron when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. a neutral chlorine atom has seven electrons in its outermost shell. Instead, it now has 17 protons and 18 electrons. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its. Chlorine Plus Electron.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Plus Electron in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. a neutral chlorine atom has seven electrons in its outermost shell. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Only one more. Chlorine Plus Electron.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Plus Electron Only one more electron is needed to achieve an octet in. a neutral chlorine atom has seven electrons in its outermost shell. Instead, it now has 17 protons and 18 electrons. It has 17 protons in the nucleus. chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic. Chlorine Plus Electron.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Plus Electron by contrast, chlorine has the electronic structure 1s 2 2s 2 2p 6 3s 2 3p x2 3p y2 3p z1. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). It. Chlorine Plus Electron.