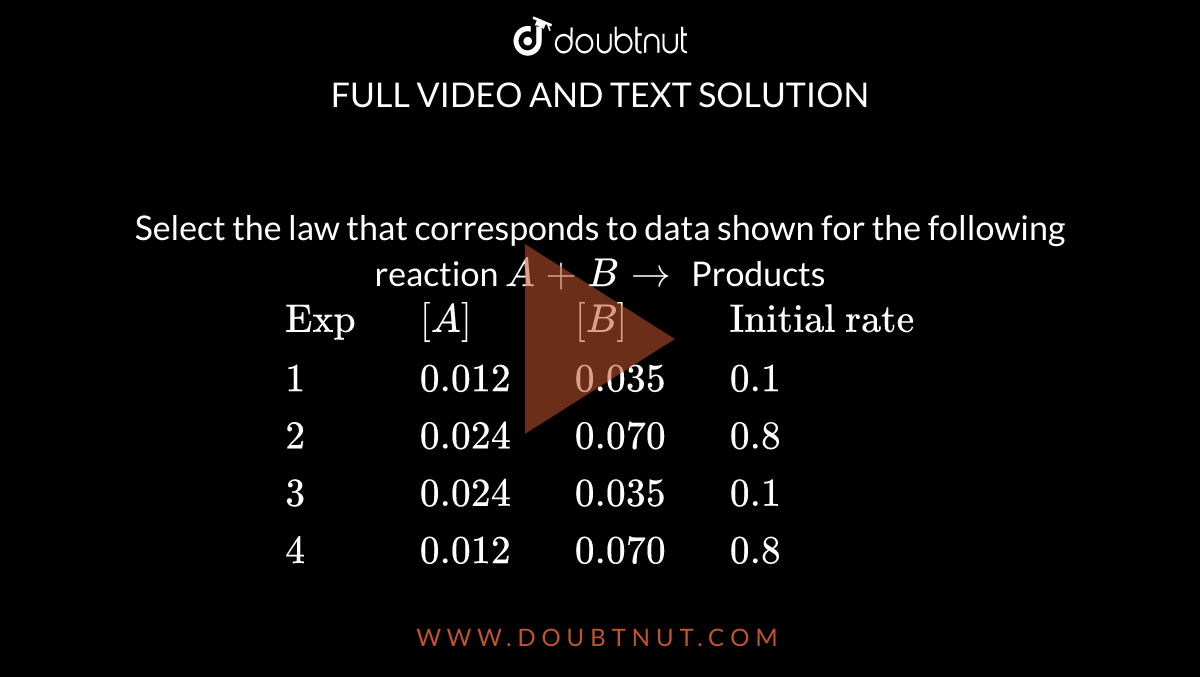
Select The Law That Corresponds To The Data . Hence, the rate law is given by the formula: A + b + c is experiment [a] m. Let the rate law be r = [a]x [b]y. ∴ 8 = (2)y , y = 3. What is the rate law that corresponds to the data shown for the reaction. Compare experiments 1 and 2 to determine the order of the reaction. (a) rate = k [b]3. Here’s how to approach this question. Chemistry ii chapter 13 quiz. Use rate and concentration data to identify reaction orders. Hence rate equation, r =. 100% (1 rating) share share. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 Select the rate law that corresponds to the data shown for the following reaction: $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and.
from www.doubtnut.com
Use rate and concentration data to identify reaction orders. What is the rate law that corresponds to the data shown for the reaction. Let the rate law be r = [a]x [b]y. Hence rate equation, r =. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Use rate laws to calculate reaction rates.
Select the law that corresponds to data shown for the following
Select The Law That Corresponds To The Data Chemistry ii chapter 13 quiz. Hence, the rate law is given by the formula: Use rate laws to calculate reaction rates. ∴ 8 = (2)y , y = 3. Compare experiments 1 and 2 to determine the order of the reaction. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. A + b + c is experiment [a] m. Hence rate equation, r =. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Select the rate law that corresponds to the data shown for the following reaction: Explain the form and function of a rate law. 100% (1 rating) share share. Here’s how to approach this question. What is the rate law that corresponds to the data shown for the reaction. Use rate and concentration data to identify reaction orders. Let the rate law be r = [a]x [b]y.
From www.youtube.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data (a) rate = k [b]3. 100% (1 rating) share share. Explain the form and function of a rate law. Here’s how to approach this question. What is the rate law that corresponds to the data shown for the reaction. ∴ 8 = (2)y , y = 3. Compare experiments 1 and 2 to determine the order of the reaction. $r. Select The Law That Corresponds To The Data.
From www.meritnation.com
find question no 15 15 2 1 2 Select the rate law that corresponds to Select The Law That Corresponds To The Data Let the rate law be r = [a]x [b]y. (a) rate = k [b]3. Here’s how to approach this question. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 Select the rate law that corresponds to the data. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Chemistry ii chapter 13 quiz. What is the rate law that corresponds to the data shown for the reaction. Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Explain the form and function of a rate law. (a) rate = k [b]3. 100% (1 rating) share share. Let the rate law be r = [a]x. Select The Law That Corresponds To The Data.
From www.coursehero.com
[Solved] Select the graph that corresponds to a shift in the supply Select The Law That Corresponds To The Data What is the rate law that corresponds to the data shown for the reaction. Here’s how to approach this question. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Compare experiments 1 and 2 to determine the order of the reaction. The rate law corresponds to the above data is (a). Select The Law That Corresponds To The Data.
From www.meritnation.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Use rate laws to calculate reaction rates. Explain the form and function of a rate law. 100% (1 rating) share share. Hence rate equation, r =. Here’s how to approach this question. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Select the rate law that corresponds to the data shown for the following. Select The Law That Corresponds To The Data.
From www.studyxapp.com
select the set that corresponds to the relation given in the arrow Select The Law That Corresponds To The Data (a) rate = k [b]3. Hence, the rate law is given by the formula: Select the rate law that corresponds to the data shown for the following reaction: Use rate laws to calculate reaction rates. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data ∴ 8 = (2)y , y = 3. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 Select the rate law that corresponds to the data shown for the following reaction: Chemistry ii chapter 13 quiz. A +. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to the data shown for the following.. Select The Law That Corresponds To The Data A + b + c is experiment [a] m. Use rate laws to calculate reaction rates. Chemistry ii chapter 13 quiz. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. 100% (1 rating) share share. (a) rate = k [b]3. Hence rate equation, r =. Compare experiments 1 and 2 to. Select The Law That Corresponds To The Data.
From www.toppr.com
1 . Select the la the law that corresponds to data show following Select The Law That Corresponds To The Data Let the rate law be r = [a]x [b]y. Hence rate equation, r =. ∴ 8 = (2)y , y = 3. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. (a) rate = k [b]3. Hence, the. Select The Law That Corresponds To The Data.
From www.toppr.com
For a reaction A + 2B→ C + D , the following data were obtained.Excpt Select The Law That Corresponds To The Data What is the rate law that corresponds to the data shown for the reaction. Here’s how to approach this question. (a) rate = k [b]3. Let the rate law be r = [a]x [b]y. Hence, the rate law is given by the formula: Use rate laws to calculate reaction rates. The rate law corresponds to the above data is (a). Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data (a) rate = k [b]3. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 100% (1 rating) share share. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Here’s how to approach. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Explain the form and function of a rate law. Compare experiments 1 and 2 to determine the order of the reaction. ∴ 8 = (2)y , y = 3. Chemistry ii chapter 13 quiz. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the law that corresponds to the data shown the following Select The Law That Corresponds To The Data Hence rate equation, r =. Use rate and concentration data to identify reaction orders. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Let the rate law be r = [a]x [b]y. Compare experiments 1 and 2 to determine the order of the reaction. Chemistry ii chapter 13 quiz. The rate law corresponds to. Select The Law That Corresponds To The Data.
From www.toppr.com
1 . Select the la the law that corresponds to data show following Select The Law That Corresponds To The Data Here’s how to approach this question. What is the rate law that corresponds to the data shown for the reaction. A + b + c is experiment [a] m. Chemistry ii chapter 13 quiz. ∴ 8 = (2)y , y = 3. Hence, the rate law is given by the formula: Use rate laws to calculate reaction rates. Use rate. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to the data shown for the following Select The Law That Corresponds To The Data 100% (1 rating) share share. Select the rate law that corresponds to the data shown for the following reaction: Compare experiments 1 and 2 to determine the order of the reaction. Hence rate equation, r =. Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Use rate and concentration data to identify reaction orders. The. Select The Law That Corresponds To The Data.
From www.chegg.com
Solved Select the term that corresponds to each of the given Select The Law That Corresponds To The Data Hence rate equation, r =. Let the rate law be r = [a]x [b]y. Select the rate law that corresponds to the data shown for the following reaction: What is the rate law that corresponds to the data shown for the reaction. Chemistry ii chapter 13 quiz. A + b + c is experiment [a] m. Compare experiments 1 and. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 A + b + c is experiment [a] m. $r = k{\left[ a. Select The Law That Corresponds To The Data.
From www.numerade.com
SOLVED Question 5 (3 points) The shaded region under a Normal Select The Law That Corresponds To The Data The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 A + b + c is experiment [a] m. Hence rate equation, r =. Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Let. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to the data shown for the following Select The Law That Corresponds To The Data (a) rate = k [b]3. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. 100% (1 rating) share share. Use rate and concentration data to identify reaction orders. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Use rate laws to calculate reaction rates. Let the rate law be r = [a]x [b]y. Use rate and concentration data to identify reaction orders. Hence rate equation, r =. Chemistry ii chapter 13 quiz. Compare experiments 1 and 2 to determine the order of the reaction. The rate law corresponds to the above data is (a) rate = k[a][b]. Select The Law That Corresponds To The Data.
From www.doubtnut.com
Select the law that corresponds to data shown for the following Select The Law That Corresponds To The Data A + b + c is experiment [a] m. Use rate laws to calculate reaction rates. Hence rate equation, r =. Select the rate law that corresponds to the data shown for the following reaction: Here’s how to approach this question. To approach the first step of selecting the correct rate law, start by writing the general rate law expression. Select The Law That Corresponds To The Data.
From www.youtube.com
Find the rate law that corresponds to the data shown for the following Select The Law That Corresponds To The Data (a) rate = k [b]3. Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Explain the form and function of a rate law. What is the rate law that corresponds to the data shown for the reaction. To approach the first step of selecting the correct rate law, start by writing the general rate law. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data ∴ 8 = (2)y , y = 3. Select the rate law that corresponds to the data shown for the following reaction: The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 A + b + c is experiment. Select The Law That Corresponds To The Data.
From www.numerade.com
SOLVEDSelect the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Here’s how to approach this question. Let the rate law be r = [a]x [b]y. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. Hence rate equation, r =. 100% (1 rating) share share. Compare experiments 1 and. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to the data shown for the following Select The Law That Corresponds To The Data Use rate laws to calculate reaction rates. A + b + c is experiment [a] m. Use rate and concentration data to identify reaction orders. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. Hence, the rate law. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Hence rate equation, r =. Chemistry ii chapter 13 quiz. Use rate and concentration data to identify reaction orders. $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3. Select The Law That Corresponds To The Data.
From www.toppr.com
1 . Select the la the law that corresponds to data show following Select The Law That Corresponds To The Data To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. Compare experiments 1 and 2 to determine the order of the reaction. Hence, the rate law is given by the formula: $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column. Select The Law That Corresponds To The Data.
From www.youtube.com
, Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Hence, the rate law is given by the formula: $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Let the rate law be r = [a]x [b]y. Select the rate law that corresponds to the data shown for the following reaction: What is the rate law that corresponds to the data shown for the. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to data shown for the following reac.. Select The Law That Corresponds To The Data Let the rate law be r = [a]x [b]y. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 Hence rate equation, r =. ∴ 8 = (2)y , y = 3. Here’s how to approach this question. $r. Select The Law That Corresponds To The Data.
From medium.com
Probability Rules Cheat Sheet. Basic probability rules with examples Select The Law That Corresponds To The Data Compare experiments 1 and 2 to determine the order of the reaction. Here’s how to approach this question. ∴ 8 = (2)y , y = 3. Hence rate equation, r =. Explain the form and function of a rate law. Use rate and concentration data to identify reaction orders. Select the rate law that corresponds to the data shown for. Select The Law That Corresponds To The Data.
From kunduz.com
[ANSWERED] Select the rate law that corresponds to data shown for the Select The Law That Corresponds To The Data Use rate laws to calculate reaction rates. ∴ 8 = (2)y , y = 3. Compare experiments 1 and 2 to determine the order of the reaction. Use rate and concentration data to identify reaction orders. Select the rate law that corresponds to the data shown for the following reaction: $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents. Select The Law That Corresponds To The Data.
From askfilo.com
Select the rate law that corresponds to data shown for the following reac.. Select The Law That Corresponds To The Data $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Compare experiments 1 and 2 to determine the order of the reaction. (a) rate = k [b]3. Select the rate law that corresponds to the data shown for the following reaction: A + b + c is experiment [a] m. The rate law corresponds to. Select The Law That Corresponds To The Data.
From askfilo.com
(Pb. P.M.T. 1994) elect the law that corresponds to the data shown the fo.. Select The Law That Corresponds To The Data Compare experiments 1 and 2 to determine the order of the reaction. ∴ 8 = (2)y , y = 3. To approach the first step of selecting the correct rate law, start by writing the general rate law expression for the reaction, r a t e = k [a] x [b]. 100% (1 rating) share share. $r = k{\left[ a. Select The Law That Corresponds To The Data.
From www.toppr.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data $r = k{\left[ a \right]^x}{\left[ b \right]^y}$ column 1 represents experiment numbers, column 2 and. Use rate laws to calculate reaction rates. ∴ 8 = (2)y , y = 3. Select the rate law that corresponds to the data shown for the following reaction: To approach the first step of selecting the correct rate law, start by writing the general. Select The Law That Corresponds To The Data.
From www.meritnation.com
Select the rate law that corresponds to the data shown for the Select The Law That Corresponds To The Data Use rate laws to calculate reaction rates. Compare experiments 1 and 2 to determine the order of the reaction. The rate law corresponds to the above data is (a) rate = k[a][b] 3 (b) rate = k[a] 2 [b] 2 (c) rate = k[b] 3 (d) rate = k[b] 4 To approach the first step of selecting the correct rate. Select The Law That Corresponds To The Data.