Is Delta H The Same As Delta E . $\delta e$ is energy change of a system at constant volume. a negative δh means that heat flows from a system to its surroundings; $\delta h$ as is energy change of a system at constant. A positive δh means that heat flows into a. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. Enthalpy change of a reaction. delta e & h will essentially be equal when there is no change in volume. Write the relationship between δ h and δ e. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. This part i understand, but the solutions manual. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and.
from www.youtube.com
the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. A positive δh means that heat flows into a. delta e & h will essentially be equal when there is no change in volume. $\delta h$ as is energy change of a system at constant. This part i understand, but the solutions manual. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. $\delta e$ is energy change of a system at constant volume. Write the relationship between δ h and δ e. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. a negative δh means that heat flows from a system to its surroundings;
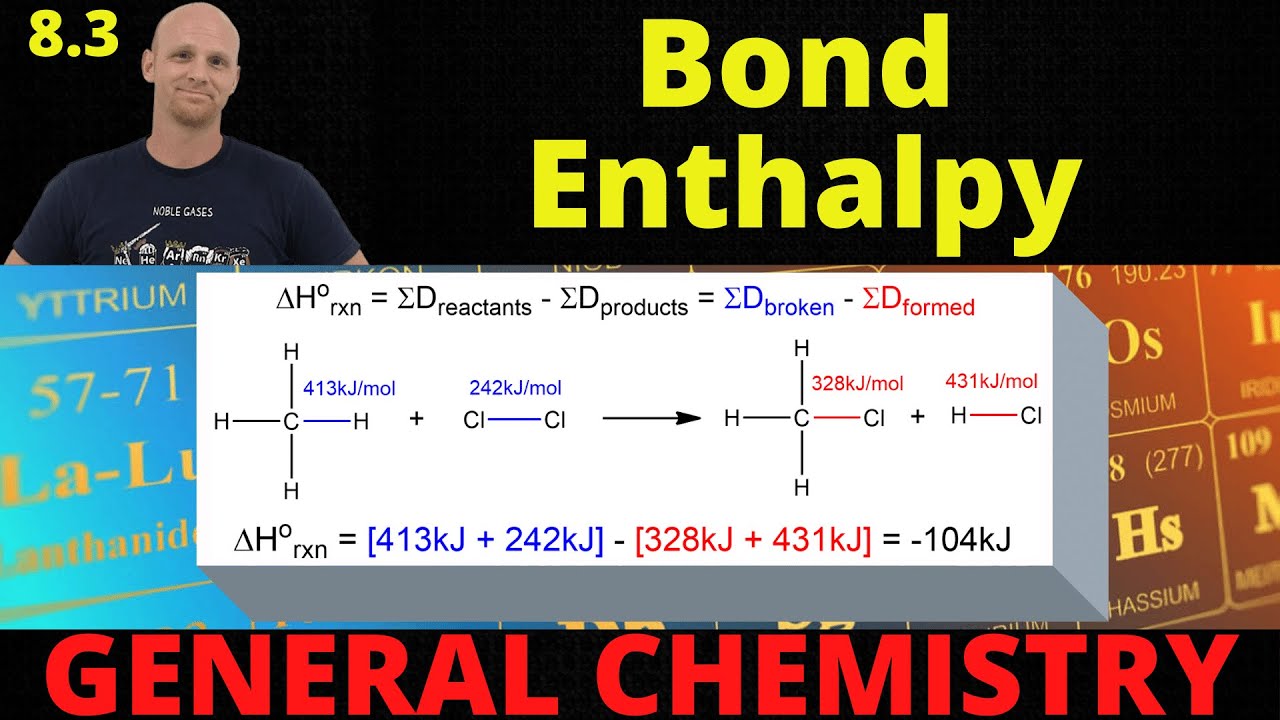
8.3 Bond Enthalpy Calculating Delta H General Chemistry YouTube
Is Delta H The Same As Delta E Enthalpy change of a reaction. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. This part i understand, but the solutions manual. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. a negative δh means that heat flows from a system to its surroundings; delta e & h will essentially be equal when there is no change in volume. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. $\delta e$ is energy change of a system at constant volume. Write the relationship between δ h and δ e. Enthalpy change of a reaction. $\delta h$ as is energy change of a system at constant. A positive δh means that heat flows into a.
From www.youtube.com
`Delta H_("fusion")` of a substance is 'x' and `Delta H_("vap")` is 'y Is Delta H The Same As Delta E Enthalpy change of a reaction. A positive δh means that heat flows into a. $\delta e$ is energy change of a system at constant volume. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. This part i understand, but the solutions manual. Write the relationship. Is Delta H The Same As Delta E.
From www.youtube.com
5.1 Delta Hf and Delta Hc calculations [SL IB Chemistry] YouTube Is Delta H The Same As Delta E A positive δh means that heat flows into a. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. $\delta e$ is energy change of a system at constant volume. a negative δh means that heat flows from a system to its surroundings; This part. Is Delta H The Same As Delta E.
From www.youtube.com
Delta H versus delta E YouTube Is Delta H The Same As Delta E $\delta e$ is energy change of a system at constant volume. a negative δh means that heat flows from a system to its surroundings; in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. A positive δh means that heat flows into a. $\delta. Is Delta H The Same As Delta E.
From brainly.in
Difference of delta h and delta e of methane at 295k Brainly.in Is Delta H The Same As Delta E delta e & h will essentially be equal when there is no change in volume. This part i understand, but the solutions manual. $\delta h$ as is energy change of a system at constant. a negative δh means that heat flows from a system to its surroundings; in a thermochemical equation, the enthalpy change of a reaction. Is Delta H The Same As Delta E.
From www.yumpu.com
Delta E, delta H, delta T What does it mean? EFI Is Delta H The Same As Delta E $\delta h$ as is energy change of a system at constant. delta e & h will essentially be equal when there is no change in volume. Enthalpy change of a reaction. a negative δh means that heat flows from a system to its surroundings; This part i understand, but the solutions manual. Write the relationship between δ h. Is Delta H The Same As Delta E.
From www.studypool.com
SOLUTION 3 CALCULATION OF DELTA E FROM DELTA H AND VICE VERSA Studypool Is Delta H The Same As Delta E in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. $\delta e$ is energy change of a system at constant volume. This part i understand, but the solutions manual. Enthalpy change of a reaction. $\delta h$ as is energy change of a system at constant.. Is Delta H The Same As Delta E.
From flexbooks.ck12.org
CK12Foundation Is Delta H The Same As Delta E A positive δh means that heat flows into a. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. This part. Is Delta H The Same As Delta E.
From www.youtube.com
Relationship of delta E with q and w YouTube Is Delta H The Same As Delta E This part i understand, but the solutions manual. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. A positive δh means that heat flows into a. $\delta h$ as is energy change of a system at constant. Write the relationship between δ h and δ e.. Is Delta H The Same As Delta E.
From schematicrasariopm.z4.web.core.windows.net
Energy Diagram For A Reaction Is Delta H The Same As Delta E Enthalpy change of a reaction. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. a negative δh means that. Is Delta H The Same As Delta E.
From www.youtube.com
Heat and Enthalpy q and delta H YouTube Is Delta H The Same As Delta E Write the relationship between δ h and δ e. $\delta e$ is energy change of a system at constant volume. delta e & h will essentially be equal when there is no change in volume. Enthalpy change of a reaction. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value. Is Delta H The Same As Delta E.
From www.youtube.com
Free energy (using delta H & delta S) YouTube Is Delta H The Same As Delta E delta e & h will essentially be equal when there is no change in volume. Enthalpy change of a reaction. $\delta e$ is energy change of a system at constant volume. Write the relationship between δ h and δ e. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference. Is Delta H The Same As Delta E.
From www.youtube.com
Enthalpy the four common ways to calculate delta H YouTube Is Delta H The Same As Delta E Enthalpy change of a reaction. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. A positive δh means that heat flows into a. $\delta e$ is energy change of a system at constant volume. Write the relationship between δ h and δ e. This part. Is Delta H The Same As Delta E.
From www.sarthaks.com
For an endothermic reaction, where `Delta H` represents the enthalpy of Is Delta H The Same As Delta E A positive δh means that heat flows into a. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. Write the relationship between δ h and δ e. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ. Is Delta H The Same As Delta E.
From www.youtube.com
8.3 Bond Enthalpy Calculating Delta H General Chemistry YouTube Is Delta H The Same As Delta E A positive δh means that heat flows into a. Enthalpy change of a reaction. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. $\delta h$ as is energy change of a system at constant. $\delta e$ is energy change of a system at constant. Is Delta H The Same As Delta E.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H The Same As Delta E delta e & h will essentially be equal when there is no change in volume. This part i understand, but the solutions manual. A positive δh means that heat flows into a. $\delta h$ as is energy change of a system at constant. Enthalpy change of a reaction. for a chemical reaction, the enthalpy of reaction (δhrxn δ. Is Delta H The Same As Delta E.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H The Same As Delta E a negative δh means that heat flows from a system to its surroundings; $\delta h$ as is energy change of a system at constant. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. A positive δh means that heat flows into a. the enthalpy. Is Delta H The Same As Delta E.
From socratic.org
How will temperature affect the spontaneity of a reaction with positive Is Delta H The Same As Delta E Write the relationship between δ h and δ e. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. $\delta e$ is energy change of a system at constant volume. $\delta h$ as is energy change of a system at constant. a. Is Delta H The Same As Delta E.
From brainly.in
give an example of reaction in which delta H equal to delta Brainly.in Is Delta H The Same As Delta E the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. Write the relationship between δ h and δ e. This part i understand, but the solutions manual. Enthalpy change of a reaction. $\delta h$ as is energy change of a system at constant. . Is Delta H The Same As Delta E.
From ar.inspiredpencil.com
Enthalpy Equation Delta H Is Delta H The Same As Delta E This part i understand, but the solutions manual. A positive δh means that heat flows into a. delta e & h will essentially be equal when there is no change in volume. a negative δh means that heat flows from a system to its surroundings; for a chemical reaction, the enthalpy of reaction (δhrxn δ h r. Is Delta H The Same As Delta E.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Is Delta H The Same As Delta E This part i understand, but the solutions manual. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. Write the relationship between δ h and δ e. a negative δh means that heat flows from a system to its surroundings; $\delta e$ is energy change. Is Delta H The Same As Delta E.
From cethunxd.blob.core.windows.net
Gas Constant Cp Cv at Luis Cox blog Is Delta H The Same As Delta E $\delta e$ is energy change of a system at constant volume. a negative δh means that heat flows from a system to its surroundings; delta e & h will essentially be equal when there is no change in volume. $\delta h$ as is energy change of a system at constant. Enthalpy change of a reaction. This part. Is Delta H The Same As Delta E.
From www.youtube.com
Calorimetry, delta E and delta H YouTube Is Delta H The Same As Delta E for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. A positive δh means that heat flows into a. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. $\delta. Is Delta H The Same As Delta E.
From imagetou.com
Como Calcular O Delta H Image to u Is Delta H The Same As Delta E This part i understand, but the solutions manual. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. A positive δh means that heat flows into a. $\delta e$ is energy change of a system at constant volume. a negative δh means. Is Delta H The Same As Delta E.
From www.numerade.com
SOLVED how to determine delta s and delta h from a graph of delta G vs T Is Delta H The Same As Delta E $\delta e$ is energy change of a system at constant volume. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. delta e & h will essentially be equal when there is no change in volume. in a thermochemical equation, the. Is Delta H The Same As Delta E.
From www.chegg.com
Solved For which of the following reactions is Delta H Is Delta H The Same As Delta E This part i understand, but the solutions manual. Enthalpy change of a reaction. for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction.. Is Delta H The Same As Delta E.
From fity.club
Enthalpy Equation Delta H Is Delta H The Same As Delta E A positive δh means that heat flows into a. a negative δh means that heat flows from a system to its surroundings; in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. $\delta h$ as is energy change of a system at constant. $\delta. Is Delta H The Same As Delta E.
From www.studypool.com
SOLUTION 3 CALCULATION OF DELTA E FROM DELTA H AND VICE VERSA Studypool Is Delta H The Same As Delta E delta e & h will essentially be equal when there is no change in volume. $\delta e$ is energy change of a system at constant volume. This part i understand, but the solutions manual. A positive δh means that heat flows into a. a negative δh means that heat flows from a system to its surroundings; . Is Delta H The Same As Delta E.
From www.youtube.com
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H Is Delta H The Same As Delta E $\delta h$ as is energy change of a system at constant. Enthalpy change of a reaction. delta e & h will essentially be equal when there is no change in volume. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. This part i understand,. Is Delta H The Same As Delta E.
From www.alpolic-americas.com
How Is Color Measured? Calculating Delta E ALPOLIC® Is Delta H The Same As Delta E $\delta h$ as is energy change of a system at constant. Enthalpy change of a reaction. delta e & h will essentially be equal when there is no change in volume. a negative δh means that heat flows from a system to its surroundings; in a thermochemical equation, the enthalpy change of a reaction is shown as. Is Delta H The Same As Delta E.
From www.youtube.com
TERMOQUÍMICA Calculando Delta H usando a LEI de HESS ou ENERGIA da Is Delta H The Same As Delta E Write the relationship between δ h and δ e. delta e & h will essentially be equal when there is no change in volume. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. for a chemical reaction, the enthalpy of reaction. Is Delta H The Same As Delta E.
From www.youtube.com
Delta H and Calorimetry YouTube Is Delta H The Same As Delta E A positive δh means that heat flows into a. a negative δh means that heat flows from a system to its surroundings; $\delta h$ as is energy change of a system at constant. Write the relationship between δ h and δ e. the enthalpy of a system can be defined in terms in terms of the internal energy,. Is Delta H The Same As Delta E.
From www.perplexity.ai
what does delta H represent Is Delta H The Same As Delta E $\delta h$ as is energy change of a system at constant. in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. a negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a. for. Is Delta H The Same As Delta E.
From www.youtube.com
Menentukan Delta H (TERMOKIMIA) KIMIA SMA YouTube Is Delta H The Same As Delta E a negative δh means that heat flows from a system to its surroundings; the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. delta e & h will essentially be equal when there is no change in volume. A positive δh means. Is Delta H The Same As Delta E.
From www.youtube.com
Konsep Dasar Perhitungan Kalor Reaksi Delta E dan Delta H YouTube Is Delta H The Same As Delta E for a chemical reaction, the enthalpy of reaction (δhrxn δ h r x n) is the difference in enthalpy between products and. the enthalpy of a system can be defined in terms in terms of the internal energy, pressure, and volume of the gas in the system. $\delta e$ is energy change of a system at constant. Is Delta H The Same As Delta E.
From www.meritnation.com
prove delta h=delta e+p delta v Chemistry Thermodynamics 16939813 Is Delta H The Same As Delta E in a thermochemical equation, the enthalpy change of a reaction is shown as a δ h value following the equation for the reaction. Enthalpy change of a reaction. This part i understand, but the solutions manual. delta e & h will essentially be equal when there is no change in volume. a negative δh means that heat. Is Delta H The Same As Delta E.