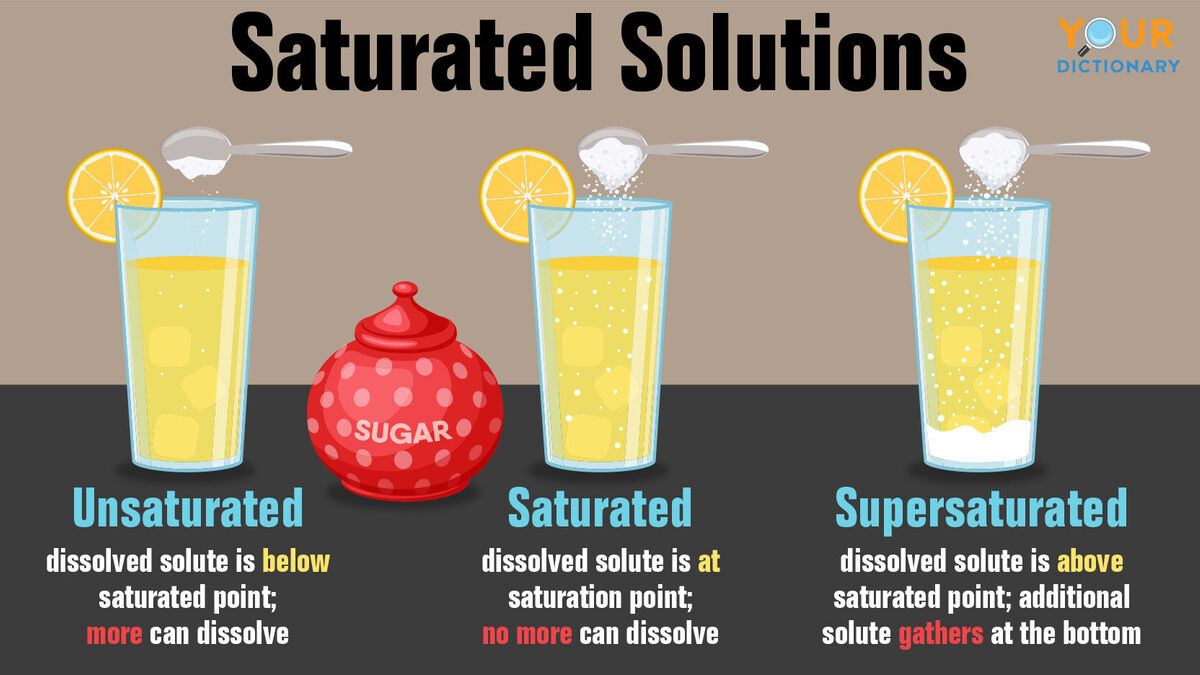
Unsaturated Solution Quizlet . a property of a solution that depends on the number, not the identity, of the solute particles. When tea can't dissolve anymore sugar. The amount of solute dissolved is large in relation to the amount of solvent present. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. distinguish between saturated and unsaturated solutions. Apply a solubility conversion factor to calculate the amount of solute that can be. solubility curve, saturation, and soluti. Explain the effects of temperature on the solubility of solids and gases. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. When tea dissolved the sugar applied. Saturated, unsaturated, supersaturated quiz for 10th grade students.
from ar.inspiredpencil.com
The amount of solute dissolved is large in relation to the amount of solvent present. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. distinguish between saturated and unsaturated solutions. When tea dissolved the sugar applied. solubility curve, saturation, and soluti. Saturated, unsaturated, supersaturated quiz for 10th grade students. a property of a solution that depends on the number, not the identity, of the solute particles. Explain the effects of temperature on the solubility of solids and gases. Apply a solubility conversion factor to calculate the amount of solute that can be.
Types Of Solutions Saturated Unsaturated And Supersaturated
Unsaturated Solution Quizlet a property of a solution that depends on the number, not the identity, of the solute particles. When tea dissolved the sugar applied. a property of a solution that depends on the number, not the identity, of the solute particles. solubility curve, saturation, and soluti. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. Explain the effects of temperature on the solubility of solids and gases. distinguish between saturated and unsaturated solutions. The amount of solute dissolved is large in relation to the amount of solvent present. Saturated, unsaturated, supersaturated quiz for 10th grade students. Apply a solubility conversion factor to calculate the amount of solute that can be. When tea can't dissolve anymore sugar. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature.
From www.coursehero.com
[Solved] Which of the following represents an unsaturated solution Unsaturated Solution Quizlet Explain the effects of temperature on the solubility of solids and gases. solubility curve, saturation, and soluti. a property of a solution that depends on the number, not the identity, of the solute particles. The amount of solute dissolved is large in relation to the amount of solvent present. distinguish between saturated and unsaturated solutions. Apply a. Unsaturated Solution Quizlet.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Unsaturated Solution Quizlet solubility curve, saturation, and soluti. When tea can't dissolve anymore sugar. Apply a solubility conversion factor to calculate the amount of solute that can be. The amount of solute dissolved is large in relation to the amount of solvent present. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature.. Unsaturated Solution Quizlet.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Solution Quizlet Saturated, unsaturated, supersaturated quiz for 10th grade students. solubility curve, saturation, and soluti. When tea can't dissolve anymore sugar. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. Explain the effects of temperature on the solubility of solids and gases. When tea dissolved the sugar applied. Apply. Unsaturated Solution Quizlet.
From www.geeksforgeeks.org
Saturated and Unsaturated Solutions Unsaturated Solution Quizlet solubility curve, saturation, and soluti. Explain the effects of temperature on the solubility of solids and gases. The amount of solute dissolved is large in relation to the amount of solvent present. Apply a solubility conversion factor to calculate the amount of solute that can be. A solution that holds as much dissolved solute as it is capable of. Unsaturated Solution Quizlet.
From byjus.com
54. explain unsaturated solution saturated solutions and supersaturated Unsaturated Solution Quizlet distinguish between saturated and unsaturated solutions. solubility curve, saturation, and soluti. Explain the effects of temperature on the solubility of solids and gases. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. When tea dissolved the sugar applied. Apply a solubility conversion factor to calculate the. Unsaturated Solution Quizlet.
From www.thoughtco.com
What Is an Unsaturated Solution in Chemistry? Unsaturated Solution Quizlet a property of a solution that depends on the number, not the identity, of the solute particles. solubility curve, saturation, and soluti. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. When tea can't dissolve anymore sugar. The amount of solute dissolved is large in relation. Unsaturated Solution Quizlet.
From www.scribd.com
2 POGIL Saturated and Unsaturated Solutions and Solubility KEY Unsaturated Solution Quizlet solubility curve, saturation, and soluti. distinguish between saturated and unsaturated solutions. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. a property of a solution that depends on the number, not the identity, of the solute particles. The amount of solute dissolved is large in relation to. Unsaturated Solution Quizlet.
From quizlet.com
What are the characteristics of unsaturated fats? Quizlet Unsaturated Solution Quizlet The amount of solute dissolved is large in relation to the amount of solvent present. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. distinguish between saturated and unsaturated solutions. When tea dissolved the sugar applied. Saturated, unsaturated, supersaturated quiz for 10th grade students. When tea can't dissolve anymore. Unsaturated Solution Quizlet.
From www.pinterest.com
Unsaturated Solution Easy Science Easy science, Solutions, Science Unsaturated Solution Quizlet distinguish between saturated and unsaturated solutions. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. solubility curve, saturation, and soluti. When tea dissolved the sugar applied. The amount of solute dissolved is large in relation to the amount of solvent present. a property of a. Unsaturated Solution Quizlet.
From www.slideserve.com
PPT Solubility Do Now p.4 PowerPoint Presentation, free download Unsaturated Solution Quizlet a property of a solution that depends on the number, not the identity, of the solute particles. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. distinguish between saturated and unsaturated solutions. Explain the effects of temperature on the solubility of solids and gases. solubility curve, saturation,. Unsaturated Solution Quizlet.
From study.com
Quiz & Worksheet Features of Unsaturated Solutions Unsaturated Solution Quizlet When tea dissolved the sugar applied. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. Apply a solubility conversion factor to calculate the amount of solute that can be.. Unsaturated Solution Quizlet.
From www.numerade.com
SOLVED 'Using the triple venn diagram below compare and contrast the Unsaturated Solution Quizlet solubility curve, saturation, and soluti. The amount of solute dissolved is large in relation to the amount of solvent present. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. Apply a solubility conversion factor to calculate the amount of solute that can be. Explain the effects of. Unsaturated Solution Quizlet.
From quizizz.com
Saturated Solutions Science Quizizz Unsaturated Solution Quizlet Apply a solubility conversion factor to calculate the amount of solute that can be. solubility curve, saturation, and soluti. When tea dissolved the sugar applied. When tea can't dissolve anymore sugar. a property of a solution that depends on the number, not the identity, of the solute particles. A solution that holds as much dissolved solute as it. Unsaturated Solution Quizlet.
From quizlet.com
What is the name of the reaction that converts unsaturated f Quizlet Unsaturated Solution Quizlet Explain the effects of temperature on the solubility of solids and gases. a property of a solution that depends on the number, not the identity, of the solute particles. When tea dissolved the sugar applied. The amount of solute dissolved is large in relation to the amount of solvent present. Apply a solubility conversion factor to calculate the amount. Unsaturated Solution Quizlet.
From www.scribd.com
Grade 7 Saturated and Unsaturated Solution Worksheet PDF Unsaturated Solution Quizlet The amount of solute dissolved is large in relation to the amount of solvent present. distinguish between saturated and unsaturated solutions. solubility curve, saturation, and soluti. Saturated, unsaturated, supersaturated quiz for 10th grade students. Explain the effects of temperature on the solubility of solids and gases. Apply a solubility conversion factor to calculate the amount of solute that. Unsaturated Solution Quizlet.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution Quizlet slidesharetrick Unsaturated Solution Quizlet A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. Saturated, unsaturated, supersaturated quiz for 10th grade students. The amount of solute dissolved is large in relation to the amount of solvent present. a property of a solution that depends on the number, not the identity, of the solute particles.. Unsaturated Solution Quizlet.
From brainly.in
define unsaturated solution Brainly.in Unsaturated Solution Quizlet solubility curve, saturation, and soluti. Explain the effects of temperature on the solubility of solids and gases. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. Apply a. Unsaturated Solution Quizlet.
From www.youtube.com
Identifying properties of unsaturated solutions YouTube Unsaturated Solution Quizlet The amount of solute dissolved is large in relation to the amount of solvent present. solubility curve, saturation, and soluti. Explain the effects of temperature on the solubility of solids and gases. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. When tea dissolved the sugar applied.. Unsaturated Solution Quizlet.
From quizzmagicfarris.z21.web.core.windows.net
Saturated And Unsaturated Solution Definition Unsaturated Solution Quizlet a property of a solution that depends on the number, not the identity, of the solute particles. Saturated, unsaturated, supersaturated quiz for 10th grade students. Apply a solubility conversion factor to calculate the amount of solute that can be. distinguish between saturated and unsaturated solutions. When tea can't dissolve anymore sugar. The amount of solute dissolved is large. Unsaturated Solution Quizlet.
From quizlet.com
COX effects the oxidation of unsaturated fatty acids other t Quizlet Unsaturated Solution Quizlet when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. a property of a solution that depends on the number, not the identity, of the solute particles. Explain the effects of temperature on the solubility of solids and gases. A solution that holds as much dissolved solute as. Unsaturated Solution Quizlet.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Unsaturated Solution Quizlet Saturated, unsaturated, supersaturated quiz for 10th grade students. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. a property of a solution that depends on the number, not the identity, of the solute particles. When tea dissolved the sugar applied. When tea can't dissolve anymore sugar. Explain the effects. Unsaturated Solution Quizlet.
From edurev.in
what is difference between saturated, unsaturated and supersaturated Unsaturated Solution Quizlet Saturated, unsaturated, supersaturated quiz for 10th grade students. When tea can't dissolve anymore sugar. Explain the effects of temperature on the solubility of solids and gases. The amount of solute dissolved is large in relation to the amount of solvent present. distinguish between saturated and unsaturated solutions. when less than the maximum amount of solute is dissolved in. Unsaturated Solution Quizlet.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution slidesharetrick Unsaturated Solution Quizlet The amount of solute dissolved is large in relation to the amount of solvent present. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. Saturated, unsaturated, supersaturated quiz for 10th grade students. distinguish between saturated and unsaturated solutions. a property of a solution that depends on the number,. Unsaturated Solution Quizlet.
From learningmagicdaniela88.z21.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Quizlet A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. When tea dissolved the sugar applied. Explain the effects of temperature on the solubility of solids and gases. solubility curve, saturation, and soluti. The amount of solute dissolved is large in relation to the amount of solvent present. distinguish. Unsaturated Solution Quizlet.
From one.wkkf.org
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Quizlet Explain the effects of temperature on the solubility of solids and gases. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. When tea dissolved the sugar applied. solubility curve, saturation, and soluti. The amount of solute dissolved is large in relation to the amount of solvent present.. Unsaturated Solution Quizlet.
From learningdbehrlichmann.z21.web.core.windows.net
Saturated And Unsaturated Solutions Worksheet Unsaturated Solution Quizlet Saturated, unsaturated, supersaturated quiz for 10th grade students. distinguish between saturated and unsaturated solutions. a property of a solution that depends on the number, not the identity, of the solute particles. Explain the effects of temperature on the solubility of solids and gases. A solution that holds as much dissolved solute as it is capable of holding stably. Unsaturated Solution Quizlet.
From www.merchantnavydecoded.com
What is saturated, unsaturated and supersaturated solution? Unsaturated Solution Quizlet Apply a solubility conversion factor to calculate the amount of solute that can be. Saturated, unsaturated, supersaturated quiz for 10th grade students. Explain the effects of temperature on the solubility of solids and gases. distinguish between saturated and unsaturated solutions. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution. Unsaturated Solution Quizlet.
From quizzmagicfarris.z21.web.core.windows.net
Saturated And Unsaturated Solutions Grade 7 Unsaturated Solution Quizlet when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. Explain the effects of temperature on the solubility of solids and gases. solubility curve, saturation, and soluti. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. Apply a. Unsaturated Solution Quizlet.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution Quizlet slidesharetrick Unsaturated Solution Quizlet When tea can't dissolve anymore sugar. a property of a solution that depends on the number, not the identity, of the solute particles. Saturated, unsaturated, supersaturated quiz for 10th grade students. The amount of solute dissolved is large in relation to the amount of solvent present. when less than the maximum amount of solute is dissolved in a. Unsaturated Solution Quizlet.
From www.chegg.com
Solved Model 1 Saturated and unsaturated Solutions Unsaturated Solution Quizlet when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. a property of a solution that depends on the number, not the identity, of the solute particles. Saturated, unsaturated, supersaturated quiz for 10th grade students. solubility curve, saturation, and soluti. Explain the effects of temperature on the. Unsaturated Solution Quizlet.
From quizzmagicfarris.z21.web.core.windows.net
Saturated And Unsaturated Solution Definition Unsaturated Solution Quizlet solubility curve, saturation, and soluti. distinguish between saturated and unsaturated solutions. Apply a solubility conversion factor to calculate the amount of solute that can be. a property of a solution that depends on the number, not the identity, of the solute particles. when less than the maximum amount of solute is dissolved in a given amount. Unsaturated Solution Quizlet.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation ID2121794 Unsaturated Solution Quizlet Saturated, unsaturated, supersaturated quiz for 10th grade students. Explain the effects of temperature on the solubility of solids and gases. The amount of solute dissolved is large in relation to the amount of solvent present. a property of a solution that depends on the number, not the identity, of the solute particles. When tea can't dissolve anymore sugar. . Unsaturated Solution Quizlet.
From ar.inspiredpencil.com
Unsaturated Solution Unsaturated Solution Quizlet when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. The amount of solute dissolved is large in relation to the amount of solvent present. a property of a solution that depends on the number, not the identity, of the solute particles. When tea can't dissolve anymore sugar.. Unsaturated Solution Quizlet.
From quizzmagicfarris.z21.web.core.windows.net
Saturated And Unsaturated Solutions Answers Unsaturated Solution Quizlet When tea dissolved the sugar applied. Apply a solubility conversion factor to calculate the amount of solute that can be. A solution that holds as much dissolved solute as it is capable of holding stably at a given temperature. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated.. Unsaturated Solution Quizlet.
From quizlet.com
Mixtures and Solutions Diagram Quizlet Unsaturated Solution Quizlet Explain the effects of temperature on the solubility of solids and gases. Apply a solubility conversion factor to calculate the amount of solute that can be. When tea dissolved the sugar applied. When tea can't dissolve anymore sugar. when less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. . Unsaturated Solution Quizlet.