Bromine Valence Electrons Atom . 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has seven valence electrons. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Iron is a transition metal, so the number. Also, we will provide the pictures of the same. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.
from ar.inspiredpencil.com
93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has seven valence electrons. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. But for most of the transition. Also, we will provide the pictures of the same.
Atomic Structure Of Bromine
Bromine Valence Electrons Atom In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Iron is a transition metal, so the number. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But for most of the transition. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Also, we will provide the pictures of the same. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Also, we will provide the pictures of the same. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In this article today we. Bromine Valence Electrons Atom.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Bromine Valence Electrons Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Bromine has seven valence electrons. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Iron. Bromine Valence Electrons Atom.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free Bromine Valence Electrons Atom Also, we will provide the pictures of the same. Iron is a transition metal, so the number. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Bromine Valence Electrons Atom.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Valence Electrons Atom Iron is a transition metal, so the number. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Bromine Valence Electrons Atom.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Valence Electrons Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Bromine Valence Electrons Atom.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Also, we will provide the pictures of the same. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence. Bromine Valence Electrons Atom.
From brainly.in
bromine valence electrons, Brainly.in Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Iron is a transition metal, so the number. Also, we will provide the pictures of the. Bromine Valence Electrons Atom.
From www.bigstockphoto.com
Bromine Atom Image & Photo (Free Trial) Bigstock Bromine Valence Electrons Atom Iron is a transition metal, so the number. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Bromine has seven valence electrons. But for most of the transition. Also, we will provide the pictures of the same. The electron configuration of an iron atom is 1s 2. Bromine Valence Electrons Atom.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Valence Electrons Atom In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. 93 rows you may assume the. Bromine Valence Electrons Atom.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Valence Electrons Atom Bromine has seven valence electrons. Iron is a transition metal, so the number. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s. Bromine Valence Electrons Atom.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Valence Electrons Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Iron is a transition. Bromine Valence Electrons Atom.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Valence Electrons Atom But for most of the transition. Also, we will provide the pictures of the same. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine. Bromine Valence Electrons Atom.
From www.pngwing.com
Electron configuration Electron shell Atom Bromine, Selfintroduction Bromine Valence Electrons Atom Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Iron is a transition metal,. Bromine Valence Electrons Atom.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Valence Electrons Atom Also, we will provide the pictures of the same. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine has. Bromine Valence Electrons Atom.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Iron is a transition metal, so the number. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 93 rows. Bromine Valence Electrons Atom.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Iron is a transition metal, so the number. But for most of the transition. Bromine has. Bromine Valence Electrons Atom.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Valence Electrons Atom Also, we will provide the pictures of the same. Iron is a transition metal, so the number. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 7 only the electrons in the outmost shell are valance electrons.all but seven of the. Bromine Valence Electrons Atom.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Bromine Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. But for most of the transition. Bromine has seven valence electrons. Iron is a. Bromine Valence Electrons Atom.
From utedzz.blogspot.com
Bromine Periodic Table Square Periodic Table Timeline Bromine Valence Electrons Atom Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Also, we will provide the pictures of the same. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons. Bromine Valence Electrons Atom.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Valence Electrons Atom Also, we will provide the pictures of the same. Bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. 7 only the electrons in the outmost shell are valance electrons.all but seven of. Bromine Valence Electrons Atom.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Valence Electrons Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. In this article today we are going to tell you about. Bromine Valence Electrons Atom.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Valence Electrons Atom Also, we will provide the pictures of the same. Iron is a transition metal, so the number. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The electron configuration of an iron atom is 1s 2 2s. Bromine Valence Electrons Atom.
From exooeohnk.blob.core.windows.net
Bromine Total Number Of Valence Electrons at Rebeca Henshaw blog Bromine Valence Electrons Atom Iron is a transition metal, so the number. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell. Bromine Valence Electrons Atom.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Also, we will provide the pictures of the same. Iron is. Bromine Valence Electrons Atom.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Bromine Valence Electrons Atom In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. But for most of the transition. Bromine has seven valence electrons. Also, we will provide the pictures of the same. The total number of electrons present in the valence shell of an atom are called valence electrons, and. Bromine Valence Electrons Atom.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Valence Electrons Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Iron is a transition metal, so the number. In this article. Bromine Valence Electrons Atom.
From joiglahlj.blob.core.windows.net
Electron Configuration Of Bromine at Johanna Brewton blog Bromine Valence Electrons Atom Also, we will provide the pictures of the same. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. But for. Bromine Valence Electrons Atom.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Valence Electrons Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. 93 rows. Bromine Valence Electrons Atom.
From www.alamy.com
Bromine Atom Shell Stock Vector Image & Art Alamy Bromine Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Also, we will provide the pictures of the same. Bromine has seven valence electrons. But for most of the transition. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2. Bromine Valence Electrons Atom.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Valence Electrons Atom Iron is a transition metal, so the number. Also, we will provide the pictures of the same. But for most of the transition. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Bromine Valence Electrons Atom.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Iron is a transition metal, so the number. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. The electron configuration of an iron. Bromine Valence Electrons Atom.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Valence Electrons Atom In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Bromine has seven valence electrons. The total number of electrons present in the valence shell of. Bromine Valence Electrons Atom.
From www.slideserve.com
PPT Atoms & Chemical Bonding PowerPoint Presentation, free download Bromine Valence Electrons Atom 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a transition metal, so. Bromine Valence Electrons Atom.
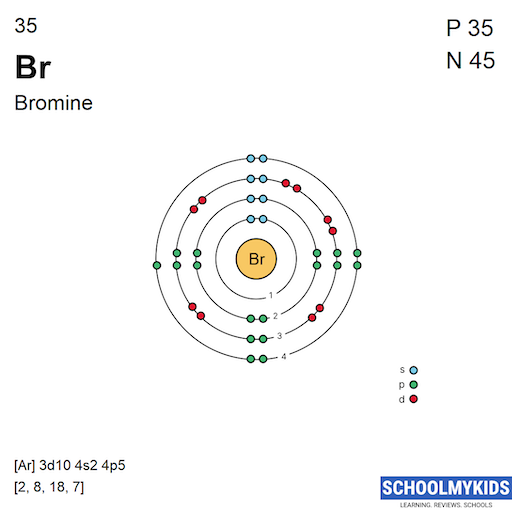
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Valence Electrons Atom In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Bromine Valence Electrons Atom.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Valence Electrons Atom Iron is a transition metal, so the number. 7 only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower shells. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Bromine Valence Electrons Atom.