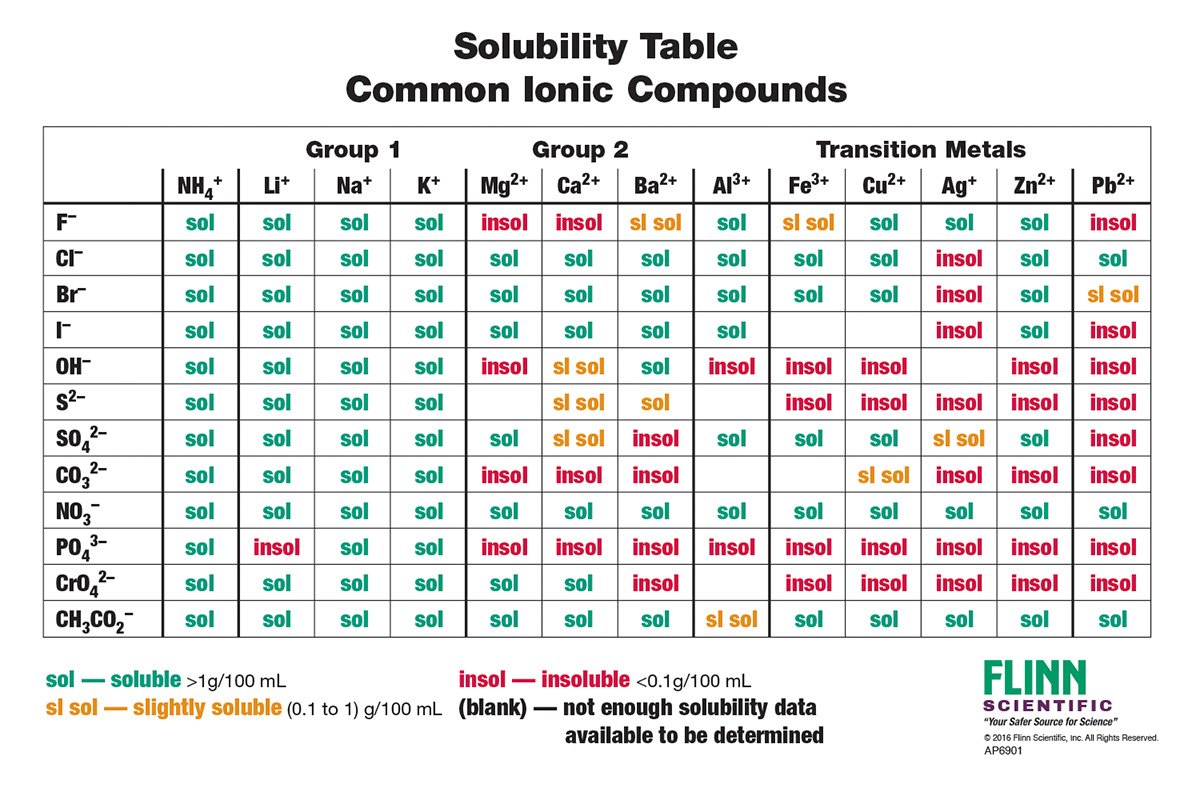
Lead Ii Nitrate Soluble Or Insoluble . Salts containing the ammonium ion. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well.
from loekjikcc.blob.core.windows.net
It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Salts containing the ammonium ion. Exceptions to this rule are rare. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well.
Lead Carbonate Solubility In Organic Solvents at Gina Miller blog
Lead Ii Nitrate Soluble Or Insoluble This reaction is characterized by the release of brown nitrogen dioxide gas. Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Salts containing the ammonium ion. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead Ii Nitrate Soluble Or Insoluble According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It describes the formation of. Lead Ii Nitrate Soluble Or Insoluble.
From brainly.in
The salt lead(II) chloride is insoluble in cold water, whereas the salt lead(II) nitrate is Lead Ii Nitrate Soluble Or Insoluble Salts containing the ammonium ion. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). This reaction is characterized by the release of brown nitrogen dioxide gas. Exceptions to this rule are rare. This page looks at the formation. Lead Ii Nitrate Soluble Or Insoluble.
From www.mingyuanchemical.com
Lead(II) nitrate 10099748 Lead Ii Nitrate Soluble Or Insoluble This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Salts containing the ammonium ion. Exceptions to this rule are rare. According to. Lead Ii Nitrate Soluble Or Insoluble.
From www.savemyexams.com
Preparing an Insoluble Salt Edexcel GCSE Chemistry Revision Notes 2018 Lead Ii Nitrate Soluble Or Insoluble This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. Exceptions to this rule are rare. Salts containing the ammonium ion. It describes the formation. Lead Ii Nitrate Soluble Or Insoluble.
From www.numerade.com
What is the mass of insoluble lead(II) iodide (461.0 g/mol) produced from 0.830 g of potassium Lead Ii Nitrate Soluble Or Insoluble This reaction is characterized by the release of brown nitrogen dioxide gas. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Exceptions to this rule are rare. According to the solubility rules table, cesium nitrate is soluble. Lead Ii Nitrate Soluble Or Insoluble.
From www.numerade.com
SOLVED Determine if the compounds will be insoluble or soluble 1. sodium nitrate 2. lead (II Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Salts containing the ammonium ion. Exceptions to this rule are rare. This reaction is. Lead Ii Nitrate Soluble Or Insoluble.
From klaalfdoh.blob.core.windows.net
Lead (Ii) Nitrate Reacts With Sodium Phosphate at James McCord blog Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This page looks at the formation of some. Lead Ii Nitrate Soluble Or Insoluble.
From socratic.org
Which of the following substances are insoluble in water? Socratic Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). This reaction is characterized by the release of brown nitrogen dioxide gas. Exceptions to this rule are rare. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. According to the solubility rules table, cesium nitrate is soluble because. Lead Ii Nitrate Soluble Or Insoluble.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). This reaction is characterized by the release of brown nitrogen dioxide gas. According to. Lead Ii Nitrate Soluble Or Insoluble.
From www.chegg.com
Solved 9. lead (II) nitrate + barium nitrate 10. Icad (II) Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. Salts containing the ammonium ion. This reaction is characterized by the release of brown nitrogen dioxide gas. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. According to the solubility rules. Lead Ii Nitrate Soluble Or Insoluble.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This page looks at the formation of some insoluble lead(ii). Lead Ii Nitrate Soluble Or Insoluble.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium hydroxide Precipitation Lead Ii Nitrate Soluble Or Insoluble According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Exceptions to this rule are rare. This page looks at. Lead Ii Nitrate Soluble Or Insoluble.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. Exceptions to this rule are rare. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This page looks. Lead Ii Nitrate Soluble Or Insoluble.
From www.alibaba.com
Super High Purity 99.0lead Ii Nitrate Msds,Cas10099748 Buy Lead Nitrate,Lead Nitrate Msds Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This reaction is characterized by the release of brown nitrogen dioxide gas. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion,. Lead Ii Nitrate Soluble Or Insoluble.
From www.artofit.org
Solubility rules chart and memorization tips Artofit Lead Ii Nitrate Soluble Or Insoluble This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Salts containing the ammonium ion. When. Lead Ii Nitrate Soluble Or Insoluble.
From www.numerade.com
SOLVED PRELAB QUESTIONS 1. Classify the following ionic compounds as soluble or insoluble in Lead Ii Nitrate Soluble Or Insoluble Salts containing the ammonium ion. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. This page looks at the formation. Lead Ii Nitrate Soluble Or Insoluble.
From joicffnpl.blob.core.windows.net
Lead Ii Nitrate Reaction With Potassium Iodide at Matilda Lee blog Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the. Lead Ii Nitrate Soluble Or Insoluble.
From joicffnpl.blob.core.windows.net
Lead Ii Nitrate Reaction With Potassium Iodide at Matilda Lee blog Lead Ii Nitrate Soluble Or Insoluble When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. Salts containing the ammonium ion. This reaction is characterized by the release of brown nitrogen dioxide gas. Exceptions to this rule are rare. According to the solubility rules table, cesium nitrate. Lead Ii Nitrate Soluble Or Insoluble.
From slideplayer.com
Unit 11 Solutions and Ions in Aqueous Solutions ppt download Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well.. Lead Ii Nitrate Soluble Or Insoluble.
From www.coursehero.com
[Solved] Problem 2 Solubility of Lead(II) Nitrate in Water The solubility... Course Hero Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation. Lead Ii Nitrate Soluble Or Insoluble.
From www.chegg.com
Solved The precipitation reaction of lead(II) nitrate with Lead Ii Nitrate Soluble Or Insoluble This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing the ammonium ion. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii). Lead Ii Nitrate Soluble Or Insoluble.
From loekjikcc.blob.core.windows.net
Lead Carbonate Solubility In Organic Solvents at Gina Miller blog Lead Ii Nitrate Soluble Or Insoluble This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing the ammonium ion. It describes. Lead Ii Nitrate Soluble Or Insoluble.
From www.chegg.com
Solved a sodium nitrate O soluble insoluble b lead(II) Lead Ii Nitrate Soluble Or Insoluble This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. Exceptions to this rule are rare. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Salts containing the ammonium. Lead Ii Nitrate Soluble Or Insoluble.
From www.sciencephoto.com
Lead (II) nitrate, Pb(NO3)2 Stock Image C030/7163 Science Photo Library Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing group i elements are soluble (li +, na +, k +,. Lead Ii Nitrate Soluble Or Insoluble.
From www.youtube.com
Insoluble salts lead(II) iodide YouTube Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. According to the solubility rules table, cesium nitrate is soluble because all compounds. Lead Ii Nitrate Soluble Or Insoluble.
From www.ceramic-glazes.com
Lead(II) nitrate is used in the manufacture of matches and special explosives such as lead azide Lead Ii Nitrate Soluble Or Insoluble Salts containing the ammonium ion. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). Exceptions to this rule are rare. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. It describes the formation of. Lead Ii Nitrate Soluble Or Insoluble.
From www.quarkochem.com
Lead (II) nitrate Quarko Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing the ammonium ion. According to the solubility rules table, cesium nitrate is soluble because all compounds containing. Lead Ii Nitrate Soluble Or Insoluble.
From www.numerade.com
SOLVED Determine if the compounds will be insoluble or soluble 1. sodium nitrate 2. lead (II Lead Ii Nitrate Soluble Or Insoluble This reaction is characterized by the release of brown nitrogen dioxide gas. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Exceptions to this rule are rare. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). According to the solubility rules table, cesium. Lead Ii Nitrate Soluble Or Insoluble.
From www.tokopedia.com
Jual LEAD (II) NITRATE/ TIMBAL (II) NITRATE/ PB(NO3)2, 500 GR, RRC Jakarta Timur eduscientia Lead Ii Nitrate Soluble Or Insoluble It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Salts containing group i elements are soluble (li +,. Lead Ii Nitrate Soluble Or Insoluble.
From slideplayer.com
Ch 18 Notes II Precipitation Reactions. Solubility Rules The solubility rules will tell you if a Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This reaction is characterized by the release of brown nitrogen dioxide gas. It describes the formation of lead(ii) hydroxide, lead(ii). Lead Ii Nitrate Soluble Or Insoluble.
From joiseczpp.blob.core.windows.net
Is Zinc Iodide Soluble Or Insoluble In Water at Marie McCandless blog Lead Ii Nitrate Soluble Or Insoluble Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. Exceptions to this rule are rare. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing the ammonium ion. When. Lead Ii Nitrate Soluble Or Insoluble.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Lead Ii Nitrate Soluble Or Insoluble According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii) iodide and lead(ii) sulfate. This page looks at the formation of some insoluble lead(ii). Lead Ii Nitrate Soluble Or Insoluble.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii) nitrate Precipitation Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen dioxide, and oxygen. This reaction is characterized by the release of brown nitrogen dioxide gas. It describes the formation of lead(ii) hydroxide, lead(ii) chloride, lead(ii). Lead Ii Nitrate Soluble Or Insoluble.
From www.ebay.com
1M Lead (Ii) Nitrate Solution, 500mL The Curated Chemical Collection eBay Lead Ii Nitrate Soluble Or Insoluble Salts containing the ammonium ion. According to the solubility rules table, cesium nitrate is soluble because all compounds containing the nitrate ion, as well. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Exceptions to this rule are rare. When you heat lead nitrate, it decomposes to form lead(ii) oxide, nitrogen. Lead Ii Nitrate Soluble Or Insoluble.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Lead Ii Nitrate Soluble Or Insoluble Exceptions to this rule are rare. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. This reaction is characterized by the release of brown nitrogen dioxide gas. Salts containing group i elements are soluble (li +, na +, k +, cs +, rb +). According to the solubility rules table, cesium. Lead Ii Nitrate Soluble Or Insoluble.