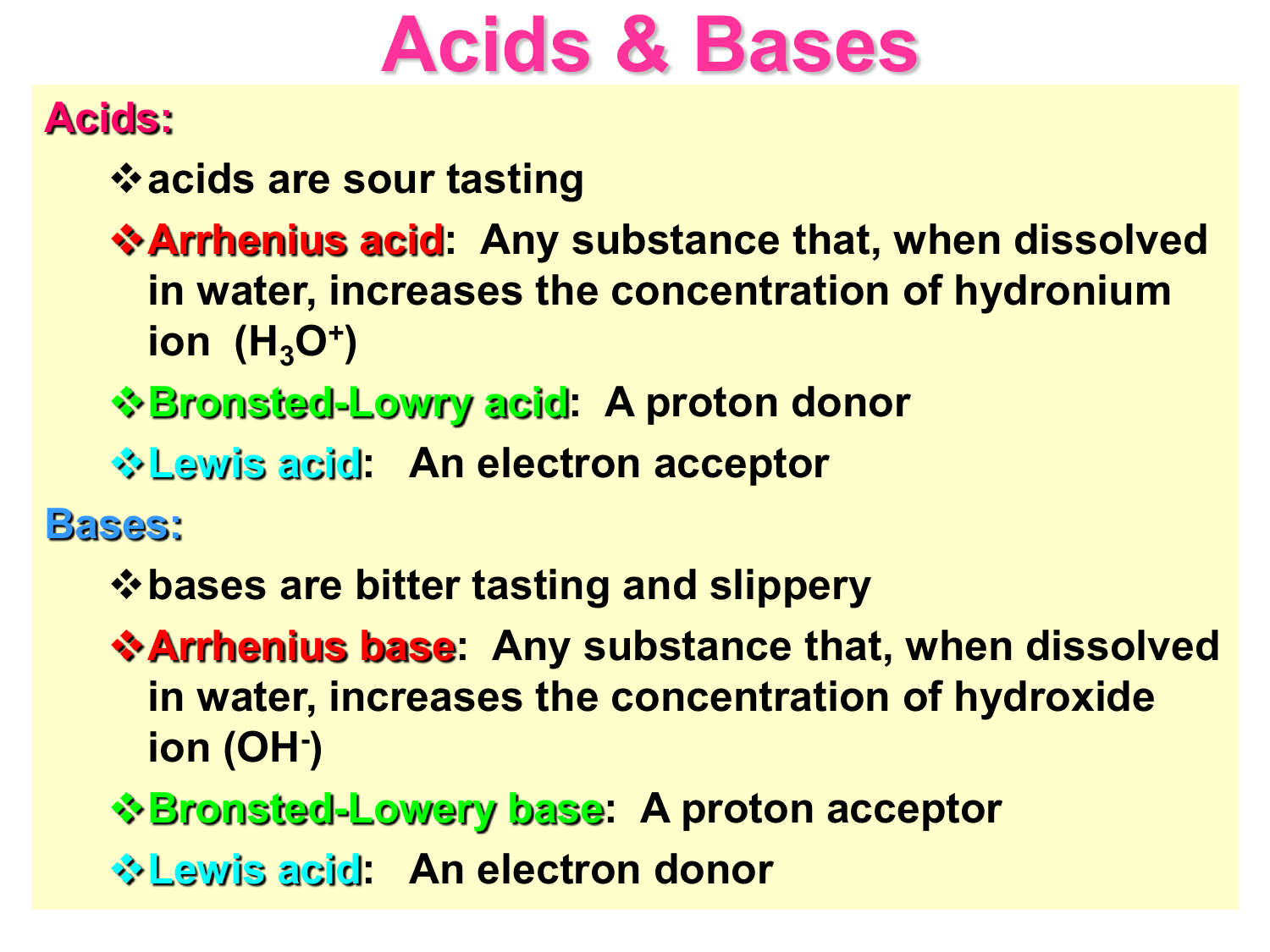
An Acid Or Base . The earliest definition of acids and bases is arrhenius's definition which states that: Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids are defined as compounds that donate a. Acids and bases can be described using the arrhenius model: 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. What's the difference between acid and base? Not to confuse you, but. Bases are the chemical opposite of acids. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Conventionally, an acid used to be known as the. Instead of protons, this lewis definition describes what molecules do with their electrons. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a.
from studylib.net
Bases are the chemical opposite of acids. Conventionally, an acid used to be known as the. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Instead of protons, this lewis definition describes what molecules do with their electrons. Acids are defined as compounds that donate a. Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids and bases can be described using the arrhenius model: The earliest definition of acids and bases is arrhenius's definition which states that:
AcidBase Chemistry
An Acid Or Base An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Not to confuse you, but. Instead of protons, this lewis definition describes what molecules do with their electrons. Bases are the chemical opposite of acids. Acids are defined as compounds that donate a. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. What's the difference between acid and base? Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Conventionally, an acid used to be known as the. Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids and bases can be described using the arrhenius model:
From www.chemicals.co.uk
What Is a Base in Chemistry? The Chemistry Blog An Acid Or Base Bases are the chemical opposite of acids. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an acid used to be known as the. Acids are defined as compounds that donate a. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Instead of. An Acid Or Base.
From www.dreamstime.com
Acids Bases and Salts Infographic Diagram Stock Vector Illustration An Acid Or Base An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acids and bases can be described using the arrhenius model: 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an acid used to be known as the. Acids. An Acid Or Base.
From mavink.com
Acid Base Map An Acid Or Base Conventionally, an acid used to be known as the. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Not to confuse you, but. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acids may be defined as the compounds that donate an ion of. An Acid Or Base.
From studylib.net
AcidBase Chemistry An Acid Or Base An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Not to confuse you, but. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. What's the difference between acid and base? In fact, a lewis acid doesn’t need to. An Acid Or Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry An Acid Or Base Acids and bases can be described using the arrhenius model: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Scientists sometimes use another scheme — the lewis system — to define acids and bases. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Bases. An Acid Or Base.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler An Acid Or Base In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Conventionally, an acid used to be known as the. The earliest definition of acids and bases is arrhenius's definition which states that: Acids and bases can be described using the arrhenius model: Not to confuse you, but. Bases are the chemical opposite of acids. 34 rows. An Acid Or Base.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses An Acid Or Base What's the difference between acid and base? Not to confuse you, but. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Instead of protons, this lewis definition describes what molecules do with their electrons. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Acids. An Acid Or Base.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases An Acid Or Base Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). The earliest definition of acids and bases is arrhenius's definition which states that: Acids are defined as compounds that donate a. Instead of protons, this lewis definition describes what molecules do with their electrons. 34 rows use this acids. An Acid Or Base.
From quizzschooltalliates.z21.web.core.windows.net
How To Identify Acids And Bases An Acid Or Base Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids and bases can be described using the arrhenius model: Conventionally, an acid used to be known as the. Instead of protons, this lewis definition describes what molecules do with their electrons. Not to confuse you, but. The earliest definition of acids and bases is. An Acid Or Base.
From www.ck12.org
Acids and Bases CK12 Foundation An Acid Or Base Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids and bases can be described using the arrhenius model: Conventionally, an acid used to be known as the. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Instead of protons, this lewis definition. An Acid Or Base.
From www.teachoo.com
Difference between Acid and Base (in Table form) Teachoo An Acid Or Base The earliest definition of acids and bases is arrhenius's definition which states that: What's the difference between acid and base? Acids and bases can be described using the arrhenius model: Acids are defined as compounds that donate a. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an. An Acid Or Base.
From sciencenotes.org
Facts About Acids and Bases An Acid Or Base Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Not to confuse you, but. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Conventionally, an acid used to be known as the. Acids and bases can be described using the arrhenius model: 34. An Acid Or Base.
From studylib.net
Acid and Base An Acid Or Base Scientists sometimes use another scheme — the lewis system — to define acids and bases. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. What's the difference between acid and base? The earliest definition of acids and bases is arrhenius's definition which states that: Conventionally, an acid used to. An Acid Or Base.
From laceynewsmiranda.blogspot.com
Enter the Conjugate Base for Each Acid. An Acid Or Base An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Acids are defined as compounds that donate a. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an acid used to be known as the. In fact, a. An Acid Or Base.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base An Acid Or Base Bases are the chemical opposite of acids. Acids are defined as compounds that donate a. What's the difference between acid and base? Instead of protons, this lewis definition describes what molecules do with their electrons. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Acids may be defined as the compounds that donate an ion. An Acid Or Base.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base An Acid Or Base 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an acid used to be known as the. Bases are the chemical opposite of acids. What's the difference between acid and base? Instead of protons, this lewis definition describes what molecules do with their electrons. In fact, a lewis. An Acid Or Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry An Acid Or Base Acids are defined as compounds that donate a. The earliest definition of acids and bases is arrhenius's definition which states that: Conventionally, an acid used to be known as the. Instead of protons, this lewis definition describes what molecules do with their electrons. Not to confuse you, but. Scientists sometimes use another scheme — the lewis system — to define. An Acid Or Base.
From topblogtenz.com
How to tell if something is an Acid or Base or Salt or Neutral? All An Acid Or Base Instead of protons, this lewis definition describes what molecules do with their electrons. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Conventionally, an acid used to be known as the. The earliest definition of acids and bases is arrhenius's definition which states that: Acids may be defined as the compounds that donate an ion. An Acid Or Base.
From www.chemistrysteps.com
Organic Acids and Bases Chemistry Steps An Acid Or Base Bases are the chemical opposite of acids. Acids and bases can be described using the arrhenius model: Not to confuse you, but. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). An acid is a substance that forms hydrogen ions h + when dissolved in water, and a. An Acid Or Base.
From byjus.com
Difference between Acid and Base Differences btw Acid & Base in An Acid Or Base Bases are the chemical opposite of acids. Not to confuse you, but. Instead of protons, this lewis definition describes what molecules do with their electrons. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Acids and bases can be described using the arrhenius model: Conventionally, an acid used. An Acid Or Base.
From www.chemistrysteps.com
Organic Acids and Bases Chemistry Steps An Acid Or Base Bases are the chemical opposite of acids. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Acids and bases can be described using the arrhenius model: In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. An acid is a substance that forms hydrogen ions. An Acid Or Base.
From www.youtube.com
Acidbase properties of salts Acids and bases AP Chemistry Khan An Acid Or Base What's the difference between acid and base? Instead of protons, this lewis definition describes what molecules do with their electrons. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Not to confuse you, but. The earliest definition of acids and bases is arrhenius's definition which states that: Conventionally, an acid used to be known as. An Acid Or Base.
From www.worksheetsplanet.com
Acid and Bases Differences An Acid Or Base Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). The earliest definition of acids and bases is arrhenius's definition which states that: Bases are the chemical opposite of acids. Conventionally, an acid used to be known as the. Instead of protons, this lewis definition describes what molecules do. An Acid Or Base.
From studylib.net
Acid and Base Summary 1 An Acid Or Base What's the difference between acid and base? 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Bases are the chemical opposite of acids. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Acids are defined as compounds that donate a. Acids may be defined. An Acid Or Base.
From www.learnatnoon.com
What are the differences between Acids and Bases? Noon An Acid Or Base 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Conventionally, an acid used to be known as the. Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another. An Acid Or Base.
From evulpo.com
Acids and bases Chemistry Explanation & Exercises evulpo An Acid Or Base In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Acids and bases can be described using the arrhenius model: Bases are the chemical opposite of acids. Not to confuse you, but. Instead of protons, this lewis definition describes what molecules do with their electrons. An acid is a substance that forms hydrogen ions h +. An Acid Or Base.
From www.thoughtco.com
10 Common Acids and Chemical Structures An Acid Or Base Conventionally, an acid used to be known as the. Acids are defined as compounds that donate a. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition. An Acid Or Base.
From pediaa.com
Difference Between Acid and Base An Acid Or Base Acids are defined as compounds that donate a. Scientists sometimes use another scheme — the lewis system — to define acids and bases. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Not to confuse you, but. In fact, a lewis acid doesn’t need to contain any hydrogen. An Acid Or Base.
From sciencenotes.org
AcidBase Chemistry An Acid Or Base Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Acids are defined as compounds that donate a. Acids and bases can be described using the arrhenius model: 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. What's. An Acid Or Base.
From www.dreamstime.com
Acid base reaction stock vector. Illustration of chemistry 200906334 An Acid Or Base Conventionally, an acid used to be known as the. What's the difference between acid and base? An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. The earliest definition of acids and bases is arrhenius's definition which states that: Acids are defined as compounds that donate a. In fact, a. An Acid Or Base.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs An Acid Or Base 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. The earliest definition of acids and bases is arrhenius's definition which states that: Instead of protons, this lewis definition describes what molecules do with their electrons.. An Acid Or Base.
From study.com
How to Identify an AcidBase Reaction Chemistry An Acid Or Base Bases are the chemical opposite of acids. In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Not to confuse you, but. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Acids are defined as compounds that donate a. What's the difference between acid and. An Acid Or Base.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry An Acid Or Base Conventionally, an acid used to be known as the. Bases are the chemical opposite of acids. Scientists sometimes use another scheme — the lewis system — to define acids and bases. Instead of protons, this lewis definition describes what molecules do with their electrons. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is. An Acid Or Base.
From kidspressmagazine.com
Acids and Bases An Acid Or Base What's the difference between acid and base? The earliest definition of acids and bases is arrhenius's definition which states that: In fact, a lewis acid doesn’t need to contain any hydrogen atoms at all. Acids may be defined as the compounds that donate an ion of hydrogen (h+) to another compound (usually called a base). Acids are defined as compounds. An Acid Or Base.
From www.youtube.com
Acids and Bases Basic Introduction Chemistry YouTube An Acid Or Base Acids are defined as compounds that donate a. Not to confuse you, but. Bases are the chemical opposite of acids. 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Acids and bases can be described using the arrhenius model: Instead of protons, this lewis definition describes what molecules do. An Acid Or Base.