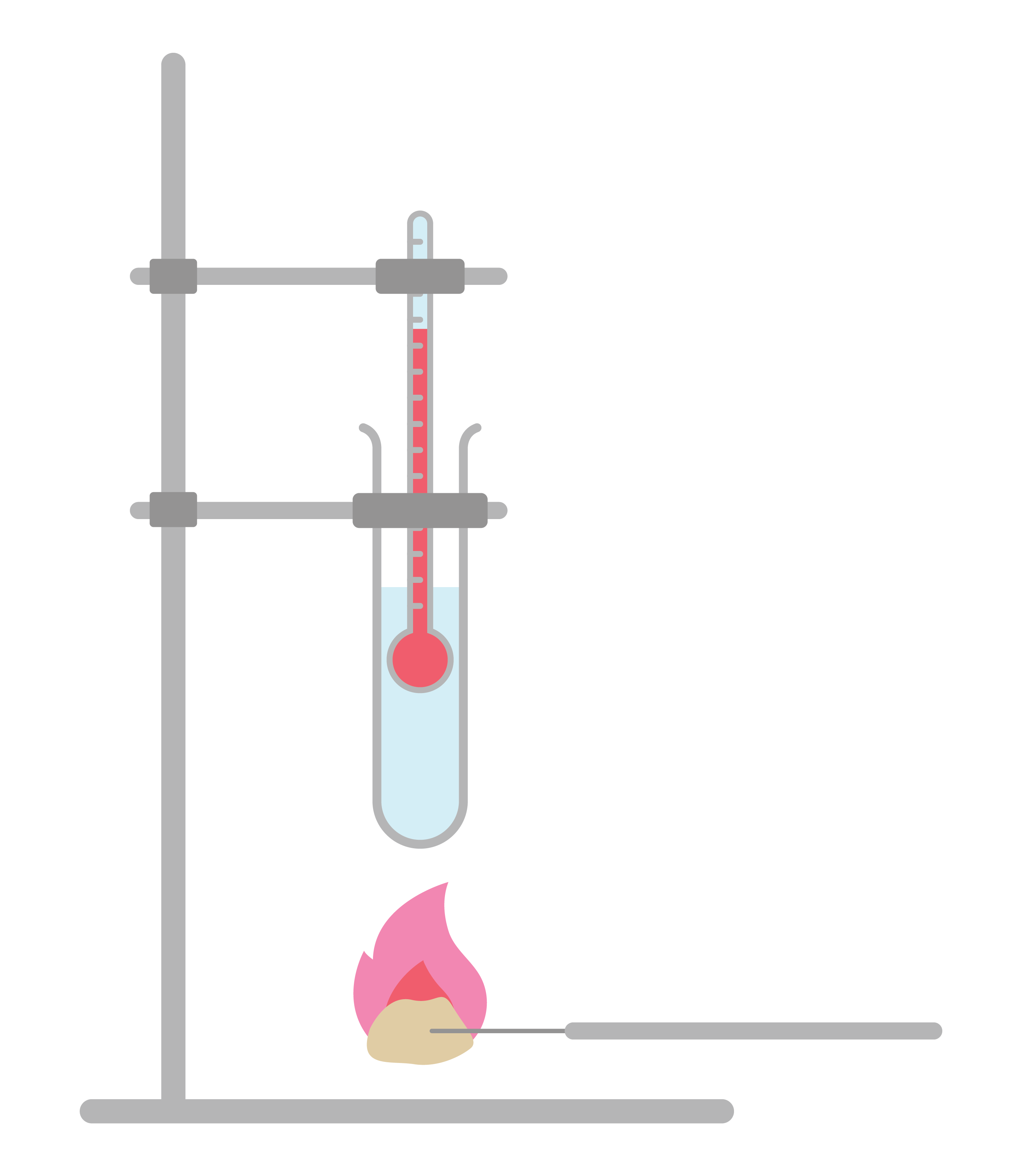
Food Calorimetry Experiment . In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Calculate the energy released by this food type using this equation: Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. Try this class experiment to investigate how much energy different foods contain. Food calorimetry allows us to determine the number of calories per gram of food. Use a calorimeter to determine the number of calories in 3 samples of food. Analyze the various types of materials by which food are made of. Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. We will determine the energy. It explains the different types of foods that are capable of generating heat and energy. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. In this activity, a piece of food is burned and the.
from evulpo.com
Use a calorimeter to determine the number of calories in 3 samples of food. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Food calorimetry allows us to determine the number of calories per gram of food. Try this class experiment to investigate how much energy different foods contain. We will determine the energy. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. In this activity, a piece of food is burned and the. It explains the different types of foods that are capable of generating heat and energy. Analyze the various types of materials by which food are made of.
evulpo Calorimetry
Food Calorimetry Experiment Try this class experiment to investigate how much energy different foods contain. Calculate the energy released by this food type using this equation: It explains the different types of foods that are capable of generating heat and energy. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Food calorimetry allows us to determine the number of calories per gram of food. In this activity, a piece of food is burned and the. We will determine the energy. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Try this class experiment to investigate how much energy different foods contain. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Use a calorimeter to determine the number of calories in 3 samples of food. Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Analyze the various types of materials by which food are made of.
From www.youtube.com
Food Calorimetry Lab YouTube Food Calorimetry Experiment Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. We will determine the energy. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Try this class experiment to. Food Calorimetry Experiment.
From www.youtube.com
Calorimetry Energy Content of Food YouTube Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Try this class experiment to investigate how much energy different foods contain. It explains the different types of foods that are capable of generating heat and energy. Different food samples can be burned in a simple calorimetry. Food Calorimetry Experiment.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Experiment We will determine the energy. Food calorimetry allows us to determine the number of calories per gram of food. Calculate the energy released by this food type using this equation: In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. In this activity, a piece of food is burned and the.. Food Calorimetry Experiment.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Food Calorimetry Experiment We will determine the energy. It explains the different types of foods that are capable of generating heat and energy. Use a calorimeter to determine the number of calories in 3 samples of food. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Try this class experiment to investigate how much energy different foods. Food Calorimetry Experiment.
From dxosivrpk.blob.core.windows.net
Food Calorimetry Lab Procedure at Ralph Safford blog Food Calorimetry Experiment It explains the different types of foods that are capable of generating heat and energy. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Food calorimetry allows. Food Calorimetry Experiment.
From www.youtube.com
Energy in Foods Calorimetry Lab YouTube Food Calorimetry Experiment In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Use a calorimeter to determine the number of calories in 3 samples of food. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Different food samples can be burned in a simple calorimetry experiment to. Food Calorimetry Experiment.
From studylib.net
experiment 9 Thermochemistry Calorimetry Food Calorimetry Experiment Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. In this activity, a piece of food is burned and the. Measure the amount of chemical energy stored in food by. Food Calorimetry Experiment.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Experiment We will determine the energy. In this activity, a piece of food is burned and the. Analyze the various types of materials by which food are made of. Try this class experiment to investigate how much energy different foods contain. Food calorimetry allows us to determine the number of calories per gram of food. From the measured temperature change, students. Food Calorimetry Experiment.
From www.vernier.com
Investigating the Energy Content of Foods > Experiment 6 from Food Calorimetry Experiment Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. We will determine the energy. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents. Food Calorimetry Experiment.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Food Calorimetry Experiment Food calorimetry allows us to determine the number of calories per gram of food. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Measure the amount of chemical energy stored in. Food Calorimetry Experiment.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Experiment It explains the different types of foods that are capable of generating heat and energy. We will determine the energy. Analyze the various types of materials by which food are made of. Use a calorimeter to determine the number of calories in 3 samples of food. From the measured temperature change, students calculate the energy transferred to the water, and. Food Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Food Calorimetry Experiment Use a calorimeter to determine the number of calories in 3 samples of food. Calculate the energy released by this food type using this equation: Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Try this class experiment to investigate how much energy. Food Calorimetry Experiment.
From evulpo.com
evulpo Calorimetry Food Calorimetry Experiment Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Calculate the energy released by this food type using this equation: Analyze the various types of materials by which food are made of. From the measured temperature change, students calculate the energy transferred to. Food Calorimetry Experiment.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Food Calorimetry Experiment Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Calculate the energy released by this food type using this equation: We will determine the energy. In this activity,. Food Calorimetry Experiment.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Food Calorimetry Experiment In this activity, a piece of food is burned and the. Try this class experiment to investigate how much energy different foods contain. We will determine the energy. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. Food calorimetry allows us to determine the number of calories per gram of. Food Calorimetry Experiment.
From dxosivrpk.blob.core.windows.net
Food Calorimetry Lab Procedure at Ralph Safford blog Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Use a calorimeter to determine the number of calories in 3 samples of food. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. In this activity, a. Food Calorimetry Experiment.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Experiment Use a calorimeter to determine the number of calories in 3 samples of food. We will determine the energy. Try this class experiment to investigate how much energy different foods contain. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Calculate the energy released by this food type using this. Food Calorimetry Experiment.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Try this class experiment to investigate how much energy different foods contain. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. Food calorimetry allows us to determine. Food Calorimetry Experiment.
From www.youtube.com
Calorimetry Experiment Demonstration YouTube Food Calorimetry Experiment Use a calorimeter to determine the number of calories in 3 samples of food. We will determine the energy. Analyze the various types of materials by which food are made of. It explains the different types of foods that are capable of generating heat and energy. Try this class experiment to investigate how much energy different foods contain. In this. Food Calorimetry Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Food Calorimetry Experiment Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Analyze the various types of materials by which food are made of. In this activity, a piece of food is burned and the. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit. Food Calorimetry Experiment.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Food Calorimetry Experiment In this activity, a piece of food is burned and the. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. It explains the different types of foods that are capable of generating heat and energy.. Food Calorimetry Experiment.
From www.chegg.com
Solved 2 A student performed a food calorimetry experiment Food Calorimetry Experiment In this activity, a piece of food is burned and the. Analyze the various types of materials by which food are made of. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It explains the different types of foods that are capable of generating heat and energy. We will determine. Food Calorimetry Experiment.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Food Calorimetry Experiment Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Food calorimetry allows us to determine the number of calories per gram of food. Analyze the various types of materials by which food are made of. Calculate the energy released by this food type using this equation: Construct a model to illustrate the flow of. Food Calorimetry Experiment.
From exysipkrj.blob.core.windows.net
How Does A Calorimeter Measure The Heat Content In Food at Carlos Hodge Food Calorimetry Experiment Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Try this class experiment to investigate how much energy different foods contain. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. We will determine. Food Calorimetry Experiment.
From www.youtube.com
Calorimetry Experiment YouTube Food Calorimetry Experiment In this activity, a piece of food is burned and the. Calculate the energy released by this food type using this equation: Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Use a calorimeter to determine the number of calories in 3 samples. Food Calorimetry Experiment.
From dxosivrpk.blob.core.windows.net
Food Calorimetry Lab Procedure at Ralph Safford blog Food Calorimetry Experiment Use a calorimeter to determine the number of calories in 3 samples of food. In this activity, a piece of food is burned and the. Food calorimetry allows us to determine the number of calories per gram of food. It explains the different types of foods that are capable of generating heat and energy. Different food samples can be burned. Food Calorimetry Experiment.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Food Calorimetry Experiment In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Try this class experiment to investigate how much energy different foods contain. It explains the different types of foods that are capable of generating heat and. Food Calorimetry Experiment.
From www.youtube.com
Calculations Help for Energy in Foods Calorimetry Lab YouTube Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Try this class experiment to investigate how much energy different foods. Food Calorimetry Experiment.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Food Calorimetry Experiment Food calorimetry allows us to determine the number of calories per gram of food. Use a calorimeter to determine the number of calories in 3 samples of food. It explains the different types of foods that are capable of generating heat and energy. In this activity, a piece of food is burned and the. Analyze the various types of materials. Food Calorimetry Experiment.
From www.chegg.com
We are going to use a calorimetry experiment to Food Calorimetry Experiment In this activity, a piece of food is burned and the. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Analyze the various types of materials by which food are made of. In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. Food calorimetry allows. Food Calorimetry Experiment.
From users.highland.edu
Calorimetry Food Calorimetry Experiment Calculate the energy released by this food type using this equation: In this activity, a piece of food is burned and the. It explains the different types of foods that are capable of generating heat and energy. Food calorimetry allows us to determine the number of calories per gram of food. Construct a model to illustrate the flow of energy. Food Calorimetry Experiment.
From www.carolina.com
Food Calorimetry How to Measure Calories in Food Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Food calorimetry allows us to determine the number of calories per gram of food. Analyze the various types of materials by which food are made of. Try this class experiment to investigate how much energy different foods. Food Calorimetry Experiment.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Food Calorimetry Experiment From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of. Try this class experiment to investigate how much energy different foods contain. Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Construct a model to illustrate the flow of energy through. Food Calorimetry Experiment.
From klagasjbp.blob.core.windows.net
Online Food Calorimetry Lab at Tinch blog Food Calorimetry Experiment Energy released (j) = mass of water (g) x rise in temperature (°c) x 4.2. Use a calorimeter to determine the number of calories in 3 samples of food. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. From the measured temperature change, students calculate the energy transferred to the. Food Calorimetry Experiment.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Experiment Measure the amount of chemical energy stored in food by burning it and capturing the heat given off in a homemade calorimeter in this fun food. Analyze the various types of materials by which food are made of. In this activity, a piece of food is burned and the. We will determine the energy. Use a calorimeter to determine the. Food Calorimetry Experiment.