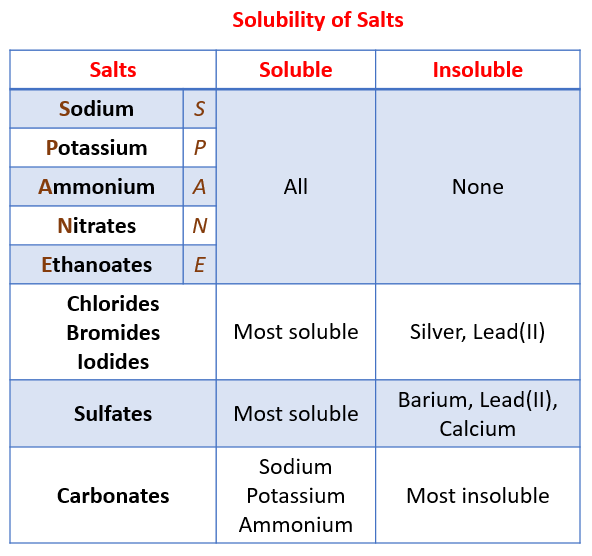
Table Salt Water Solubility . The first substance is table salt, or sodium chloride. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. Compared with other salts, agcl is poorly soluble in water. Water temperature can have a significant effect on the solubility of compounds. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. They are odorless but flavorful crystals that are white in color. It is readily soluble in water but either partially soluble or inert in other liquids. These rules are general and qualitative in nature. A table for the solubility of salts in water. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Refer to the chart below to find reference values per gram of.
from www.onlinemathlearning.com
These rules are general and qualitative in nature. A table for the solubility of salts in water. It is readily soluble in water but either partially soluble or inert in other liquids. Water temperature can have a significant effect on the solubility of compounds. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. They are odorless but flavorful crystals that are white in color. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. The first substance is table salt, or sodium chloride. Refer to the chart below to find reference values per gram of. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility.
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets
Table Salt Water Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. A table for the solubility of salts in water. Compared with other salts, agcl is poorly soluble in water. It is readily soluble in water but either partially soluble or inert in other liquids. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. They are odorless but flavorful crystals that are white in color. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. The first substance is table salt, or sodium chloride. These rules are general and qualitative in nature.
From answer-helper.com
Solubility of salts in water is temperature dependent. consider the Table Salt Water Solubility Water temperature can have a significant effect on the solubility of compounds. The first substance is table salt, or sodium chloride. A table for the solubility of salts in water. It is readily soluble in water but either partially soluble or inert in other liquids. Compared with other salts, agcl is poorly soluble in water. Refer to the chart below. Table Salt Water Solubility.
From www.sampletemplates.com
FREE 7+ Sample Solubility Chart Templates in PDF MS Word Table Salt Water Solubility The first substance is table salt, or sodium chloride. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. A table for the solubility of salts in water. Compared with other salts, agcl is poorly soluble in water. They are odorless but flavorful crystals that are. Table Salt Water Solubility.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Table Salt Water Solubility Compared with other salts, agcl is poorly soluble in water. Refer to the chart below to find reference values per gram of. These rules are general and qualitative in nature. It is readily soluble in water but either partially soluble or inert in other liquids. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room. Table Salt Water Solubility.
From www.chegg.com
Solved Arrange the salts by their molar solubility in water. Table Salt Water Solubility Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. It is readily soluble in water but either partially soluble or inert in other liquids. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. As you would. Table Salt Water Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID5581895 Table Salt Water Solubility Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. Refer to the chart below to find reference values per gram of. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. It is readily soluble in water. Table Salt Water Solubility.
From www.researchgate.net
Solubility of certain salts in water at different temperatures Table Salt Water Solubility These rules are general and qualitative in nature. Compared with other salts, agcl is poorly soluble in water. They are odorless but flavorful crystals that are white in color. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a. Table Salt Water Solubility.
From www.chegg.com
Solved The solubility of salt water is a function of the Table Salt Water Solubility Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. Compared with other salts, agcl is poorly soluble in water. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. 133 rows in this section we will apply chemical equilibria. Table Salt Water Solubility.
From ar.inspiredpencil.com
Solubility Of Salt Table Salt Water Solubility A table for the solubility of salts in water. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. It is readily soluble in water but either partially soluble or inert in other liquids. They are odorless but flavorful crystals that are white in color. The first substance is table salt, or sodium chloride. Refer to the. Table Salt Water Solubility.
From www.researchgate.net
Solubility curves of sodium salts. Download Scientific Diagram Table Salt Water Solubility The first substance is table salt, or sodium chloride. A table for the solubility of salts in water. Water temperature can have a significant effect on the solubility of compounds. It is readily soluble in water but either partially soluble or inert in other liquids. These rules are general and qualitative in nature. Due to the unrestricted mobility of the. Table Salt Water Solubility.
From www.slideserve.com
PPT Solubility of Salts PowerPoint Presentation, free download ID Table Salt Water Solubility Refer to the chart below to find reference values per gram of. Compared with other salts, agcl is poorly soluble in water. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Water temperature can have a significant effect on the solubility of compounds. These rules are general and qualitative in nature. The first. Table Salt Water Solubility.
From mavink.com
Salt Solubility Chart Table Salt Water Solubility A table for the solubility of salts in water. Compared with other salts, agcl is poorly soluble in water. Water temperature can have a significant effect on the solubility of compounds. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. 133 rows in this section we will apply chemical equilibria to the concept of solubility and. Table Salt Water Solubility.
From www.sliderbase.com
Solutions and solubility Presentation Chemistry Table Salt Water Solubility Water temperature can have a significant effect on the solubility of compounds. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Refer to the chart below to find reference values per gram of. A table for the solubility of salts in water. Due to the unrestricted mobility of the ions, nacl in its. Table Salt Water Solubility.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Table Salt Water Solubility The first substance is table salt, or sodium chloride. A table for the solubility of salts in water. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. These rules are general and qualitative in nature. It is readily soluble in water but either partially soluble or inert in other liquids. Refer to the chart below to. Table Salt Water Solubility.
From mungfali.com
Salt Solubility Chart Table Salt Water Solubility These rules are general and qualitative in nature. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Refer to the chart below to find reference values per gram of. A table for the solubility of salts in water. Water temperature can have a significant effect. Table Salt Water Solubility.
From learnchemistrysaltform4.weebly.com
Qualitative analysis of salts Learn Chemistry Corner Table Salt Water Solubility They are odorless but flavorful crystals that are white in color. Refer to the chart below to find reference values per gram of. The first substance is table salt, or sodium chloride. It is readily soluble in water but either partially soluble or inert in other liquids. Due to the unrestricted mobility of the ions, nacl in its aqueous form. Table Salt Water Solubility.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Table Salt Water Solubility The first substance is table salt, or sodium chloride. These rules are general and qualitative in nature. Water temperature can have a significant effect on the solubility of compounds. Compared with other salts, agcl is poorly soluble in water. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Table Salt Water Solubility.
From www.sliderbase.com
Precipitation Reactions Presentation Chemistry Table Salt Water Solubility They are odorless but flavorful crystals that are white in color. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. The first substance is table salt, or sodium chloride. It is readily soluble in water but either partially soluble or inert in other liquids. These rules are general and qualitative in. Table Salt Water Solubility.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Table Salt Water Solubility It is readily soluble in water but either partially soluble or inert in other liquids. Compared with other salts, agcl is poorly soluble in water. They are odorless but flavorful crystals that are white in color. Water temperature can have a significant effect on the solubility of compounds. Due to the unrestricted mobility of the ions, nacl in its aqueous. Table Salt Water Solubility.
From www.dreamstime.com
Solubility in water table stock illustration. Illustration of alkalis Table Salt Water Solubility Water temperature can have a significant effect on the solubility of compounds. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. The first substance is table salt, or sodium chloride. Refer to the chart below to find reference values per gram of. As you would. Table Salt Water Solubility.
From www.pinterest.com
Solubility Rules Chart for Chemistry Classroom 11th chemistry Table Salt Water Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. These rules are general and qualitative in nature. Refer to the chart below to find reference values per gram of. The first substance is table salt, or sodium chloride. A table for the solubility of salts. Table Salt Water Solubility.
From www.semanticscholar.org
[PDF] Solubility of NaCl, NaBr, and KCl in Water, Methanol, Ethanol Table Salt Water Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Compared with other salts, agcl is poorly soluble in water. It is readily soluble in water but either partially soluble or inert in other liquids. Water temperature can have a significant effect on the solubility of. Table Salt Water Solubility.
From sample-templates123.com
How To Read And Understand A Solubility Chart Example Free Sample Table Salt Water Solubility Refer to the chart below to find reference values per gram of. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. These rules are general and qualitative in nature. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. 133 rows in this section we will apply chemical. Table Salt Water Solubility.
From www.slideserve.com
PPT Chem. 212(01/02/03) Solubility of a Salt PowerPoint Table Salt Water Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. The first substance is table salt, or sodium chloride. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. 1 liter of water can dissolve 1.34 × 10. Table Salt Water Solubility.
From www.researchgate.net
Solubility curves of sodium salts. Download Scientific Diagram Table Salt Water Solubility They are odorless but flavorful crystals that are white in color. As you would almost certainly predict, especially if you’ve ever inadvertently taken a. Water temperature can have a significant effect on the solubility of compounds. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Refer to the chart below to find reference. Table Salt Water Solubility.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Table Salt Water Solubility Compared with other salts, agcl is poorly soluble in water. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. They are odorless but flavorful crystals that are white in color. These rules are general and qualitative in nature. A table for the solubility of salts. Table Salt Water Solubility.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Table Salt Water Solubility Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. These rules are general and qualitative in nature. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. A table for the solubility of salts in water. As. Table Salt Water Solubility.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Table Salt Water Solubility They are odorless but flavorful crystals that are white in color. A table for the solubility of salts in water. Compared with other salts, agcl is poorly soluble in water. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. The first substance is table salt, or sodium chloride. It is readily soluble in. Table Salt Water Solubility.
From stock.adobe.com
Substances solubility table Stock Vector Adobe Stock Table Salt Water Solubility 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Water temperature can have a significant effect on the solubility of compounds. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Due to the unrestricted mobility of the ions,. Table Salt Water Solubility.
From icandochemistry.com
Solubility of Salts Video] O Level Secondary Chemistry Tuition Table Salt Water Solubility These rules are general and qualitative in nature. A table for the solubility of salts in water. Compared with other salts, agcl is poorly soluble in water. They are odorless but flavorful crystals that are white in color. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. As you would almost certainly predict,. Table Salt Water Solubility.
From www.chegg.com
Solved Simple Rules for Solubility of Salts in Water (Table Table Salt Water Solubility As you would almost certainly predict, especially if you’ve ever inadvertently taken a. They are odorless but flavorful crystals that are white in color. Refer to the chart below to find reference values per gram of. These rules are general and qualitative in nature. 133 rows in this section we will apply chemical equilibria to the concept of solubility and. Table Salt Water Solubility.
From socratic.org
Which of the following substances are insoluble in water? Socratic Table Salt Water Solubility Water temperature can have a significant effect on the solubility of compounds. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Compared with other salts, agcl is poorly soluble in water. These rules are general and qualitative in nature. As you would almost certainly predict,. Table Salt Water Solubility.
From www.slideserve.com
PPT Reactions in Solution PowerPoint Presentation, free download ID Table Salt Water Solubility They are odorless but flavorful crystals that are white in color. A table for the solubility of salts in water. These rules are general and qualitative in nature. Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. Due to the unrestricted mobility of the ions,. Table Salt Water Solubility.
From sites.google.com
CP Lab Report Requirements Masco Chemistry Table Salt Water Solubility Compared with other salts, agcl is poorly soluble in water. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Due to the unrestricted mobility of the ions, nacl in its aqueous form is an excellent electrical conductor. The first substance is table salt, or sodium. Table Salt Water Solubility.
From mungfali.com
Water Solubility Table Table Salt Water Solubility Compared with other salts, agcl is poorly soluble in water. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the solubility. Water temperature can have a significant effect on the solubility. Table Salt Water Solubility.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Table Salt Water Solubility As you would almost certainly predict, especially if you’ve ever inadvertently taken a. Water temperature can have a significant effect on the solubility of compounds. 1 liter of water can dissolve 1.34 × 10 −5 moles of agcl at room temperature. Compared with other salts, agcl is poorly soluble in water. A table for the solubility of salts in water.. Table Salt Water Solubility.