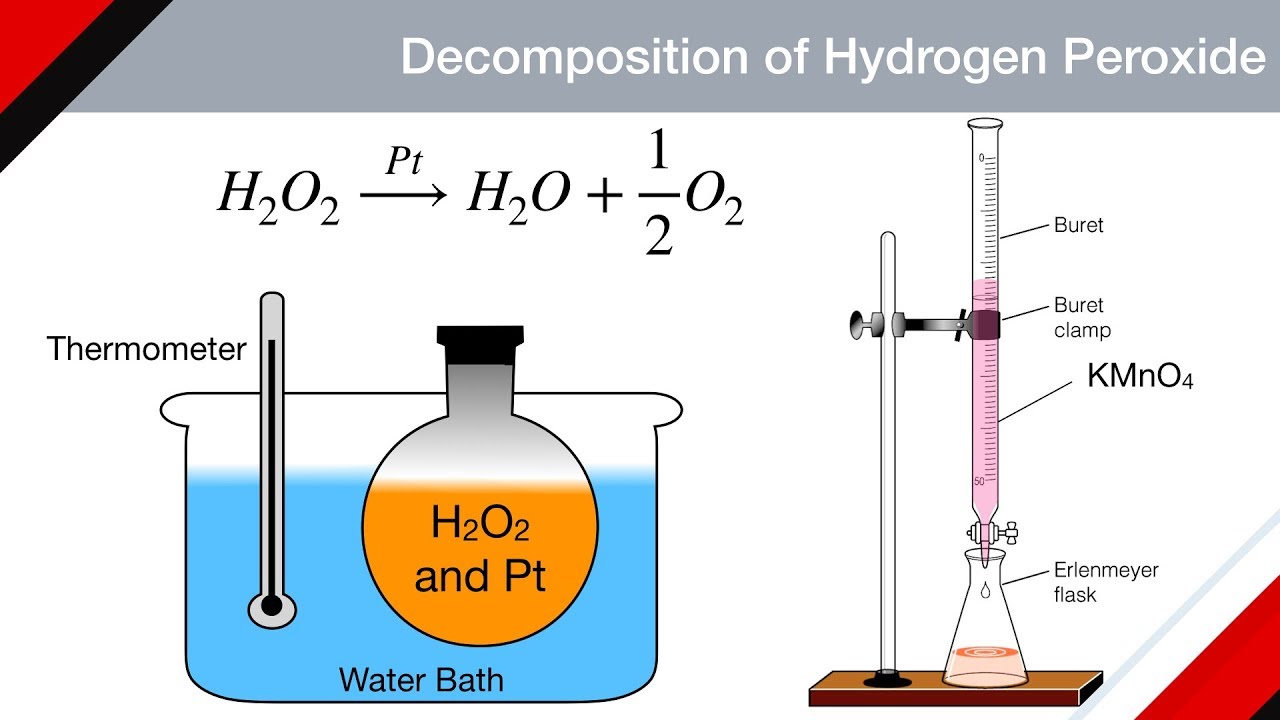
Catalytic Decomposition Of Hydrogen Peroxide Word Equation . Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Includes kit list and safety instructions. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Catalytic decomposition of hydrogen peroxide. Manganese dioxide is used to catalyse the decomposition of hydrogen. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition.
from dereonrtmontgomery.blogspot.com
The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Manganese dioxide is used to catalyse the decomposition of hydrogen. Includes kit list and safety instructions. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalytic decomposition of hydrogen peroxide. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide.
of Hydrogen Peroxide Equation DereonrtMontgomery
Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Manganese dioxide is used to catalyse the decomposition of hydrogen. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation:
From www.tessshebaylo.com
Balanced Equation For The Breakdown Of Hydrogen Peroxide By Manganese Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. Includes kit list and safety instructions. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates.. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Hydrogen Peroxide Chemical Equation Tessshebaylo Catalytic Decomposition Of Hydrogen Peroxide Word Equation Includes kit list and safety instructions. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Catalytic. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.numerade.com
SOLVED The catalytic of hydrogen peroxide yields oxygen Catalytic Decomposition Of Hydrogen Peroxide Word Equation Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Manganese dioxide is used to catalyse the decomposition of hydrogen. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.researchgate.net
Scheme II Reaction scheme for the catalytic of hydrogen Catalytic Decomposition Of Hydrogen Peroxide Word Equation To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.slideshare.net
Reactions & Formulas Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Includes kit list and safety instructions. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Hydrogen peroxide is injurious. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.numerade.com
SOLVED The catalytic of hydrogen peroxide yields oxygen Catalytic Decomposition Of Hydrogen Peroxide Word Equation The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Catalytic decomposition of hydrogen peroxide. To investigate the effect of. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Chemical Equation For Hydrogen Peroxide And Potassium Iodide Reaction Catalytic Decomposition Of Hydrogen Peroxide Word Equation The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Chemical Equation For The Reaction Between Hydrogen Peroxide And Catalytic Decomposition Of Hydrogen Peroxide Word Equation The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalytic decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Explanation. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.researchgate.net
Possible mechanism of hydrogen peroxide catalyzed by Catalytic Decomposition Of Hydrogen Peroxide Word Equation The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Manganese dioxide is used to catalyse the decomposition of hydrogen. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.chegg.com
Solved The of hydrogen peroxide, H202 , 2 H2O2 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Includes kit list and safety instructions. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Catalytic decomposition of hydrogen. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From dereonrtmontgomery.blogspot.com
of Hydrogen Peroxide Equation DereonrtMontgomery Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Hydrogen peroxide is injurious to cells. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Catalase Hydrogen Peroxide Chemical Equation Tessshebaylo Catalytic Decomposition Of Hydrogen Peroxide Word Equation Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide.. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Of Hydrogen Peroxide Into Catalytic Decomposition Of Hydrogen Peroxide Word Equation Includes kit list and safety instructions. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Manganese dioxide is used to catalyse the decomposition of hydrogen. Hydrogen peroxide is injurious to cells because it attacks unsaturated. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.sciencephoto.com
Catalytic of Hydrogen Peroxide Stock Image C030/7308 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: To investigate the effect of different solids on the. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From ar.inspiredpencil.com
Catalase Reaction With Hydrogen Peroxide Catalytic Decomposition Of Hydrogen Peroxide Word Equation The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Manganese. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.tessshebaylo.com
Hydrogen Peroxide Chemical Equation Tessshebaylo Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.flinnsci.com
Catalytic of Hydrogen Peroxide Flinn Scientific Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalytic decomposition of hydrogen peroxide. Includes kit list and safety instructions. Hydrogen peroxide is injurious to cells. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.slideserve.com
PPT Ch. 9 Chemical Reactions & Equations PowerPoint Presentation ID Catalytic Decomposition Of Hydrogen Peroxide Word Equation Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From studylib.net
of Hydrogen Peroxide Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. Manganese dioxide is used to catalyse the decomposition of hydrogen. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Hydrogen. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From pubs.acs.org
New Insights into the Mechanism of the Catalytic of Catalytic Decomposition Of Hydrogen Peroxide Word Equation The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Catalytic decomposition of hydrogen peroxide. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Manganese dioxide is used to catalyse the. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.coursehero.com
[Solved] Consider the of hydrogen peroxide reaction. If Catalytic Decomposition Of Hydrogen Peroxide Word Equation Includes kit list and safety instructions. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition.. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.sciencephoto.com
Catalytic of Hydrogen Peroxide Stock Image C030/7305 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The decomposition. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.nagwa.com
Question Video Identifying the Balanced Chemical Equation for the Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Hydrogen peroxide is injurious to cells because it attacks. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.youtube.com
Effect of Various Catalysts on Hydrogen Peroxide GCSE Chemistry YouTube Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Includes kit list and safety instructions. Catalytic decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab,. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation ID1436564 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.chegg.com
Experiment 2 of Hydrogen Peroxide The Catalytic Decomposition Of Hydrogen Peroxide Word Equation Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Collect a range of catalysts to explore. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.bartleby.com
Answered The of hydrogen peroxide… bartleby Catalytic Decomposition Of Hydrogen Peroxide Word Equation The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation: Catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Hydrogen peroxide is injurious to cells. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From opencoursewarefinance.web.fc2.com
Hydrogen peroxide chemistry help? Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Manganese dioxide is used to catalyse the decomposition of hydrogen. The decomposition of hydrogen. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From lemurianembassy.com
👍 Hydrogen peroxide word equation. chemistry. 20190224 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates.. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.youtube.com
of Hydrogen Peroxide Reactions Chemistry The Fuse Catalytic Decomposition Of Hydrogen Peroxide Word Equation Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Manganese dioxide is used to catalyse the decomposition of hydrogen. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. Catalytic decomposition of hydrogen peroxide. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.slideserve.com
PPT Air PowerPoint Presentation, free download ID1879186 Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The decomposition of hydrogen peroxide, h 2 o 2, which was demonstrated in this lab, proceeds according to the following equation:. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.vrogue.co
The Catalytic Of Hydrogen Peroxide Writ vrogue.co Catalytic Decomposition Of Hydrogen Peroxide Word Equation Catalytic decomposition of hydrogen peroxide. Manganese dioxide is used to catalyse the decomposition of hydrogen. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Includes kit list and safety instructions. Explanation (including important chemical equations) hydrogen peroxide undergoes disproportionation. To investigate the effect of different solids on the catalytic. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.chegg.com
Solved 2 H2O2 (aq) + 2 H2O(l) + O2 (g) Hydrogen peroxide, Catalytic Decomposition Of Hydrogen Peroxide Word Equation To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The decomposition of hydrogen peroxide, h 2. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From www.nagwa.com
Question Video Identifying the Correct Statement For the Catalytic Decomposition Of Hydrogen Peroxide Word Equation Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Manganese dioxide is used to catalyse the decomposition of hydrogen. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. The catalytic decomposition of hydrogen peroxide allows the use of. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.
From reubengokegoodwin.blogspot.com
of Hydrogen Peroxide Equation Catalytic Decomposition Of Hydrogen Peroxide Word Equation Manganese dioxide is used to catalyse the decomposition of hydrogen. Includes kit list and safety instructions. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids (lipids) found in cell membranes and consequently cells. Catalytic decomposition of hydrogen peroxide. To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide. The catalytic decomposition of hydrogen. Catalytic Decomposition Of Hydrogen Peroxide Word Equation.