Charge Formed Chlorine . One electron will be transferred from. You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. Chlorine is in group 7 of the periodic table. Therefore a chlorine atom has 7 electrons in its outer shell. This atom needs one more electron. Notice again how the one is left off of the. 93 rows ionic charge: The 2 subscript is in the ionic formula because we need two cl −. This electric charge generated on the ion is. The chlorine is in the form of a negatively charged ion, not the neutral element. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −.
from www.numerade.com
Chlorine is in group 7 of the periodic table. One electron will be transferred from. The chlorine is in the form of a negatively charged ion, not the neutral element. 93 rows this table shows the most common charges for atoms of the chemical elements. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). You can use this table to predict whether an atom can bond with another atom. Therefore a chlorine atom has 7 electrons in its outer shell. This electric charge generated on the ion is.
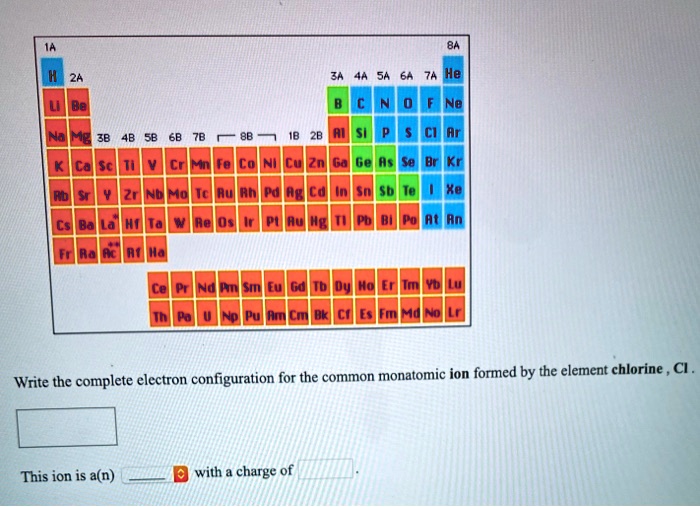
SOLVED Write the complete electron configuration for the common
Charge Formed Chlorine 93 rows ionic charge: Therefore a chlorine atom has 7 electrons in its outer shell. One electron will be transferred from. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: This electric charge generated on the ion is. The 2 subscript is in the ionic formula because we need two cl −. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Chlorine is in group 7 of the periodic table. You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows this table shows the most common charges for atoms of the chemical elements. This atom needs one more electron. Notice again how the one is left off of the. The chlorine is in the form of a negatively charged ion, not the neutral element.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Charge Formed Chlorine This electric charge generated on the ion is. This atom needs one more electron. The 2 subscript is in the ionic formula because we need two cl −. 93 rows this table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge for elements on the periodic table, in this.. Charge Formed Chlorine.
From melscience.com
Physical and chemical characteristic of chlorine and its acidic Charge Formed Chlorine Chlorine is in group 7 of the periodic table. 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows ionic charge: This electric charge generated on the ion is. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. This atom needs one more. Charge Formed Chlorine.
From brainly.com
An ion of calcium has a +2 charge, and an ion of chlorine has a 1 Charge Formed Chlorine 93 rows ionic charge: One electron will be transferred from. 93 rows this table shows the most common charges for atoms of the chemical elements. The chlorine is in the form of a negatively charged ion, not the neutral element. This atom needs one more electron. The chemical formula for a chlorine atom is cl and the chemical formula for. Charge Formed Chlorine.
From www.solutionspile.com
[Solved] 27) Predict the charge that the ion form Charge Formed Chlorine 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). One electron will be transferred from. This electric charge generated on the ion is. Using a simple, general trend for the ionic charge for elements. Charge Formed Chlorine.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Charge Formed Chlorine When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Therefore a chlorine atom has 7 electrons in its outer shell. 93 rows this table shows the most common charges for atoms of the chemical elements. One electron will be transferred from. You can use this table to predict whether. Charge Formed Chlorine.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Charge Formed Chlorine The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. This atom needs one more electron. Notice again how the one is left off of the. 93 rows ionic charge: One electron will be transferred from. Therefore a chlorine atom has 7 electrons in its outer shell. Chlorine is in group. Charge Formed Chlorine.
From www.sliderbase.com
Ions Charge Formed Chlorine The 2 subscript is in the ionic formula because we need two cl −. Therefore a chlorine atom has 7 electrons in its outer shell. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The chlorine is in the form of a negatively charged ion, not the neutral element.. Charge Formed Chlorine.
From mammothmemory.net
Ions are created when atoms lose or gain electrons Charge Formed Chlorine This electric charge generated on the ion is. You can use this table to predict whether an atom can bond with another atom. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. 93 rows ionic charge: Using a simple, general trend for the ionic charge for elements on the periodic. Charge Formed Chlorine.
From socratic.org
What are similarities of covalent and ionic bonding? Socratic Charge Formed Chlorine You can use this table to predict whether an atom can bond with another atom. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. The 2 subscript is in the ionic formula because we need two cl −. The chlorine is in the form of a negatively charged ion, not. Charge Formed Chlorine.
From www.vecteezy.com
Chlorine symbol. Chemical element of the periodic table. Vector Charge Formed Chlorine Chlorine is in group 7 of the periodic table. The chlorine is in the form of a negatively charged ion, not the neutral element. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Using a simple, general trend for the ionic charge for elements on the periodic table, in this.. Charge Formed Chlorine.
From www.chegg.com
Solved A chlorine ion has a charge of e and a mass of 5.81 Charge Formed Chlorine Notice again how the one is left off of the. The 2 subscript is in the ionic formula because we need two cl −. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. This electric charge generated on the ion is. The chlorine is in the form of a negatively. Charge Formed Chlorine.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Charge Formed Chlorine This electric charge generated on the ion is. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Therefore a chlorine atom has 7 electrons in its outer shell. When the atom loses or. Charge Formed Chlorine.
From slideplayer.com
Bundle 2 Periodic Table and Bonding ppt download Charge Formed Chlorine You can use this table to predict whether an atom can bond with another atom. Chlorine is in group 7 of the periodic table. This electric charge generated on the ion is. One electron will be transferred from. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Using a. Charge Formed Chlorine.
From chemtech-us.com
15 Interesting Facts About Chlorine Charge Formed Chlorine You can use this table to predict whether an atom can bond with another atom. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Chlorine is in group 7 of the periodic table. This atom needs one more electron. The 2 subscript is in the ionic formula because we need. Charge Formed Chlorine.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Charge Formed Chlorine This atom needs one more electron. Chlorine is in group 7 of the periodic table. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. The. Charge Formed Chlorine.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Charge Formed Chlorine One electron will be transferred from. 93 rows ionic charge: Notice again how the one is left off of the. The 2 subscript is in the ionic formula because we need two cl −. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. The chlorine is in the form of. Charge Formed Chlorine.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Charge Formed Chlorine You can use this table to predict whether an atom can bond with another atom. Chlorine is in group 7 of the periodic table. The chlorine is in the form of a negatively charged ion, not the neutral element. This atom needs one more electron. One electron will be transferred from. When the atom loses or gains one or more. Charge Formed Chlorine.
From readingandwritingprojectcom.web.fc2.com
potassium chloride ionic or covalent Charge Formed Chlorine One electron will be transferred from. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Notice again how the one is left off of the. The 2 subscript is in the ionic formula because we need two cl −. When the atom loses or gains one or more electrons, the electric charge. Charge Formed Chlorine.
From slideplayer.com
UNIT THREE BIOLOGY AREA OF STUDY 1 MOLECULES OF LIFE EXAM REVISION Charge Formed Chlorine This electric charge generated on the ion is. Therefore a chlorine atom has 7 electrons in its outer shell. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Notice again how the. Charge Formed Chlorine.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Charge Formed Chlorine The 2 subscript is in the ionic formula because we need two cl −. The chlorine is in the form of a negatively charged ion, not the neutral element. One electron will be transferred from. This electric charge generated on the ion is. Therefore a chlorine atom has 7 electrons in its outer shell. 93 rows this table shows the. Charge Formed Chlorine.
From en.wikipedia.org
Ionic bonding Wikipedia Charge Formed Chlorine Notice again how the one is left off of the. The chlorine is in the form of a negatively charged ion, not the neutral element. You can use this table to predict whether an atom can bond with another atom. This atom needs one more electron. 93 rows this table shows the most common charges for atoms of the chemical. Charge Formed Chlorine.
From www.numerade.com
SOLVED Write the complete electron configuration for the common Charge Formed Chlorine Notice again how the one is left off of the. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Using a simple, general trend for the ionic charge for elements on the periodic table, in this. The 2 subscript is in. Charge Formed Chlorine.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Charge Formed Chlorine This electric charge generated on the ion is. The 2 subscript is in the ionic formula because we need two cl −. One electron will be transferred from. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another atom. This atom. Charge Formed Chlorine.
From h-o-m-e.org
The Formal Charge in Chlorine's Most Common Form Perchlorate Charge Formed Chlorine The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. The 2 subscript is in the ionic formula because we need two cl −. Notice again how the one is left off of the. When the atom loses or gains one or more electrons, the electric charge is generated (and an. Charge Formed Chlorine.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Charge Formed Chlorine Notice again how the one is left off of the. This atom needs one more electron. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. Therefore a chlorine atom has. Charge Formed Chlorine.
From gardenandplate.com
Molecules Charge Formed Chlorine One electron will be transferred from. You can use this table to predict whether an atom can bond with another atom. Therefore a chlorine atom has 7 electrons in its outer shell. Notice again how the one is left off of the. 93 rows ionic charge: This atom needs one more electron. The chlorine is in the form of a. Charge Formed Chlorine.
From donghokiddy.com
What Are 5 Surprising Uses Of Chlorine? Charge Formed Chlorine One electron will be transferred from. You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Therefore a chlorine atom has 7 electrons in its outer shell. Notice again how the one is left off of the. Chlorine. Charge Formed Chlorine.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Charge Formed Chlorine 93 rows this table shows the most common charges for atoms of the chemical elements. The 2 subscript is in the ionic formula because we need two cl −. One electron will be transferred from. Notice again how the one is left off of the. You can use this table to predict whether an atom can bond with another atom.. Charge Formed Chlorine.
From ar.inspiredpencil.com
What Is Ion Charge Formed Chlorine 93 rows ionic charge: The chlorine is in the form of a negatively charged ion, not the neutral element. Notice again how the one is left off of the. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. The 2 subscript is in the ionic formula because we need two cl −.. Charge Formed Chlorine.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Charge Formed Chlorine This electric charge generated on the ion is. You can use this table to predict whether an atom can bond with another atom. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. The 2 subscript is in the ionic formula because we need two cl −. 93 rows this table. Charge Formed Chlorine.
From www.numerade.com
SOLVED 'Predict the charge on the most common monatomic ion formed by Charge Formed Chlorine Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Notice again how the one is left off of the. Chlorine is in group 7 of the periodic table. Therefore a chlorine atom has 7 electrons in its outer shell. When the atom loses or gains one or more electrons, the electric charge. Charge Formed Chlorine.
From www.chegg.com
Solved Chlorine forms an ion with a charge of? a. 7− b. 2+ Charge Formed Chlorine The 2 subscript is in the ionic formula because we need two cl −. You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Therefore a chlorine atom has 7. Charge Formed Chlorine.
From brainly.in
draw atomic structure of chlorine Brainly.in Charge Formed Chlorine Therefore a chlorine atom has 7 electrons in its outer shell. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This atom needs one more electron. The chlorine is in the form of a negatively charged ion, not the neutral element. Using a simple, general trend for the ionic. Charge Formed Chlorine.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Charge Formed Chlorine 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another atom. Notice again how the one is left off of the. The 2 subscript is in the ionic formula because we need two cl −. The chlorine is in the form. Charge Formed Chlorine.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Charge Formed Chlorine 93 rows ionic charge: The 2 subscript is in the ionic formula because we need two cl −. This atom needs one more electron. The chemical formula for a chlorine atom is cl and the chemical formula for the ion is cl −. You can use this table to predict whether an atom can bond with another atom. One electron. Charge Formed Chlorine.