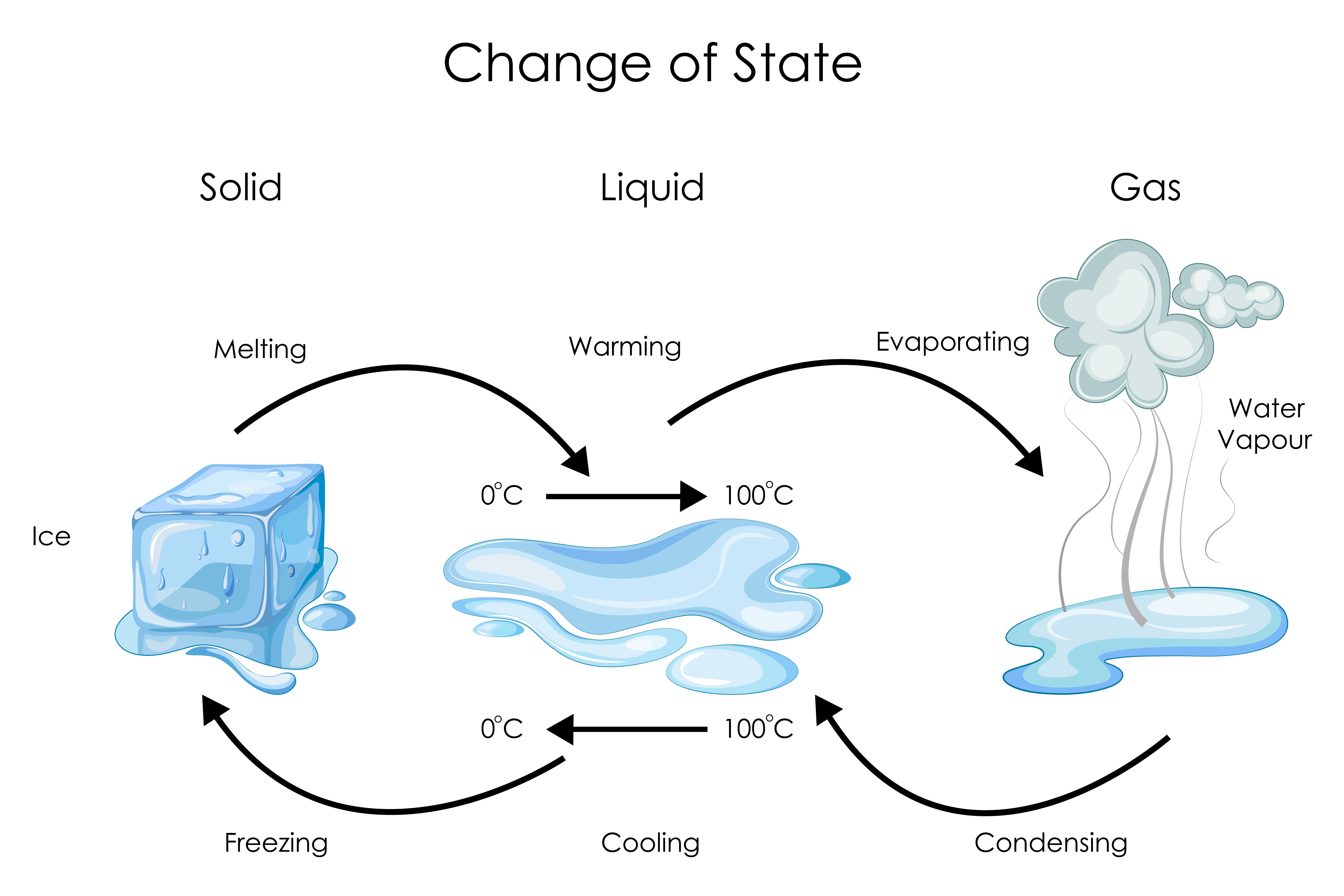
Does Kinetic Energy Increase When Ice Melts . As the ice continues to melt, the heat will continue to. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. As ice melts into water, kinetic energy is being added to the particles. When ice or any other solid melts, its potential energy increases. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. When those molecules slow down, their kinetic energy decreases. Indeed, this is the only increase in energy, since the thermal kinetic. At the melting point, they have.
from primaryleap.co.uk
Indeed, this is the only increase in energy, since the thermal kinetic. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. As ice melts into water, kinetic energy is being added to the particles. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. When ice or any other solid melts, its potential energy increases. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. At the melting point, they have.
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk
Does Kinetic Energy Increase When Ice Melts When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. Indeed, this is the only increase in energy, since the thermal kinetic. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. When those molecules slow down, their kinetic energy decreases. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. When ice or any other solid melts, its potential energy increases. At the melting point, they have. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. As ice melts into water, kinetic energy is being added to the particles. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. As the ice continues to melt, the heat will continue to.
From courses.lumenlearning.com
The First Law of Thermodynamics Biology for Majors I Does Kinetic Energy Increase When Ice Melts After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. As the ice continues to melt, the heat will continue to. When those molecules slow down, their kinetic energy decreases. As the temperature of the ice increases, the water molecules in the. Does Kinetic Energy Increase When Ice Melts.
From www.usatoday.com
Ice sheets melting at poles faster than before Does Kinetic Energy Increase When Ice Melts As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. Indeed, this is the only increase in energy, since the thermal kinetic. As the ice continues to melt, the heat will continue to. When the substance melts or boils, energy is put in to breaking the bonds. Does Kinetic Energy Increase When Ice Melts.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Does Kinetic Energy Increase When Ice Melts Indeed, this is the only increase in energy, since the thermal kinetic. At the melting point, they have. When those molecules slow down, their kinetic energy decreases. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When the substance melts or. Does Kinetic Energy Increase When Ice Melts.
From www.pbs.org
Melting to Keep Cool NOVA PBS Does Kinetic Energy Increase When Ice Melts When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. As the ice continues to melt, the heat will continue to. As ice. Does Kinetic Energy Increase When Ice Melts.
From scienceline.ucsb.edu
UCSB Science Line Does Kinetic Energy Increase When Ice Melts Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. In the case of ice melting under atmospheric pressure, the volume contraction means. Does Kinetic Energy Increase When Ice Melts.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Does Kinetic Energy Increase When Ice Melts As the ice continues to melt, the heat will continue to. At the melting point, they have. As ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. As the temperature of the ice increases, the water molecules in the. Does Kinetic Energy Increase When Ice Melts.
From opened.cuny.edu
Biology 2e, The Cell, Metabolism, The Laws of Thermodynamics OpenEd CUNY Does Kinetic Energy Increase When Ice Melts When ice or any other solid melts, its potential energy increases. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once. Does Kinetic Energy Increase When Ice Melts.
From physics.aps.org
Physics A Look at Ice Friction Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. When ice or any other solid melts, its potential energy increases. After the ice has completely melted, continued heating of the water will now increase the kinetic. Does Kinetic Energy Increase When Ice Melts.
From news.uci.edu
Canadian glaciers now major contributor to sea level change, UCI study Does Kinetic Energy Increase When Ice Melts As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. After the ice has completely melted, continued heating of the water will now increase the kinetic energy. Does Kinetic Energy Increase When Ice Melts.
From www.nytimes.com
Greenland’s Melting Ice Sheet Could Raise Sea Levels by Nearly a Foot Does Kinetic Energy Increase When Ice Melts As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. After the ice has completely melted, continued heating of the water will now. Does Kinetic Energy Increase When Ice Melts.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office Does Kinetic Energy Increase When Ice Melts As the ice continues to melt, the heat will continue to. As ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles. Does Kinetic Energy Increase When Ice Melts.
From news.ufl.edu
07 Methaneeating microbes found beneath Antarctica's melting ice Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. Indeed, this is the only increase in energy, since the thermal kinetic. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that. Does Kinetic Energy Increase When Ice Melts.
From www.nytimes.com
Climate Model Predicts West Antarctic Ice Sheet Could Melt Rapidly Does Kinetic Energy Increase When Ice Melts Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. Indeed, this is the only increase in energy, since the thermal kinetic. At the melting point, they have. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the. Does Kinetic Energy Increase When Ice Melts.
From socratic.org
What happens to the energy of its molecules as ice melts into Does Kinetic Energy Increase When Ice Melts As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the. Does Kinetic Energy Increase When Ice Melts.
From www.elevise.co.uk
P3 E) States of Matter AQA Combined Science Trilogy Elevise Does Kinetic Energy Increase When Ice Melts After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. As ice melts into water, kinetic energy is. Does Kinetic Energy Increase When Ice Melts.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Does Kinetic Energy Increase When Ice Melts As the ice continues to melt, the heat will continue to. When ice or any other solid melts, its potential energy increases. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles. Does Kinetic Energy Increase When Ice Melts.
From ian.umces.edu
Experiment Demonstrating Melting Ice Media Library Integration and Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. Indeed, this is the only increase in energy, since the thermal kinetic. Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. As the ice continues to melt, the heat will continue to. After. Does Kinetic Energy Increase When Ice Melts.
From www.bbc.com
Sealevel rise from polar ice melt finally quantified BBC News Does Kinetic Energy Increase When Ice Melts When ice or any other solid melts, its potential energy increases. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases. Does Kinetic Energy Increase When Ice Melts.
From courses.lumenlearning.com
Phase Changes Physics Does Kinetic Energy Increase When Ice Melts After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. As the ice continues to melt, the heat. Does Kinetic Energy Increase When Ice Melts.
From study.com
Polar Ice Caps Melting Causes & Impacts Lesson Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. When those molecules slow down, their kinetic energy decreases. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid. Does Kinetic Energy Increase When Ice Melts.
From www.bbc.com
Melt may explain Antarctica's sea ice expansion BBC News Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. When ice or any other solid melts, its potential energy increases. As the temperature of the ice increases, the. Does Kinetic Energy Increase When Ice Melts.
From pressbooks.online.ucf.edu
14.3 Phase Change and Latent Heat College Physics Does Kinetic Energy Increase When Ice Melts When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. When those molecules slow down, their kinetic energy decreases. As the ice continues to melt, the heat will continue to. After the ice has completely melted, continued heating of the water will now increase the kinetic. Does Kinetic Energy Increase When Ice Melts.
From www.usatoday.com
Climate change Antarctic ice melting is accelerating Does Kinetic Energy Increase When Ice Melts When those molecules slow down, their kinetic energy decreases. At the melting point, they have. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. As ice melts into water, kinetic energy is being added to the particles. Indeed, this is the only increase in energy, since. Does Kinetic Energy Increase When Ice Melts.
From socratic.org
How would you use the phase diagram of water to explain why ice at the Does Kinetic Energy Increase When Ice Melts When ice or any other solid melts, its potential energy increases. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. Indeed, this is the only increase in energy, since the thermal kinetic. As ice melts into water, kinetic energy is being added to the particles. After the ice has. Does Kinetic Energy Increase When Ice Melts.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass Does Kinetic Energy Increase When Ice Melts At the melting point, they have. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Indeed, this is the only increase. Does Kinetic Energy Increase When Ice Melts.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY Does Kinetic Energy Increase When Ice Melts In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. As the ice continues to melt, the heat will continue to. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. When those molecules slow down, their. Does Kinetic Energy Increase When Ice Melts.
From getrevising.co.uk
States of Matter Revision Cards in IGCSE Chemistry Does Kinetic Energy Increase When Ice Melts When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. When ice or any other solid melts, its potential energy increases. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will,. Does Kinetic Energy Increase When Ice Melts.
From stock.adobe.com
Types of energy MELTS scheme vector illustration. Labeled acronym Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. When those molecules slow down, their kinetic energy decreases. At the melting point, they have. As the temperature of the ice increases, the water molecules in the ice crystal absorb more and more energy and vibrate more vigorously. This causes them to be 'excited' and they break. Does Kinetic Energy Increase When Ice Melts.
From www.cnn.com
See glaciers melt before your eyes CNN Video Does Kinetic Energy Increase When Ice Melts Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. After the ice has completely melted, continued heating of the water will now. Does Kinetic Energy Increase When Ice Melts.
From www.theguardian.com
Global warming is melting Antarctic ice from below John Abraham Does Kinetic Energy Increase When Ice Melts Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. At the melting point, they have. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. This causes them to be 'excited' and. Does Kinetic Energy Increase When Ice Melts.
From www.worldwisetutoring.com
Heating and Cooling Curves Does Kinetic Energy Increase When Ice Melts Once the ice reaches its melting point the line becomes horizontal (between b and c), showing that the temperature does not increase during. Indeed, this is the only increase in energy, since the thermal kinetic. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. This. Does Kinetic Energy Increase When Ice Melts.
From www.nytimes.com
Study Predicts Antarctica Ice Melt if All Fossil Fuels Are Burned The Does Kinetic Energy Increase When Ice Melts After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. As the ice continues to melt, the heat will continue to. At the melting point, they have. When ice or any other solid melts, its potential energy increases. As the temperature of. Does Kinetic Energy Increase When Ice Melts.
From socratic.org
If you are wearing wet clothes, and the water evaporates, it cools you Does Kinetic Energy Increase When Ice Melts This causes them to be 'excited' and they break the bonds that hold them together as a solid,. As ice melts into water, kinetic energy is being added to the particles. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases the potential energy. When those molecules slow down,. Does Kinetic Energy Increase When Ice Melts.
From www.baamboozle.com
Potential and Energy Review Baamboozle Baamboozle The Does Kinetic Energy Increase When Ice Melts At the melting point, they have. This causes them to be 'excited' and they break the bonds that hold them together as a solid,. After the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. In the case of ice melting under atmospheric. Does Kinetic Energy Increase When Ice Melts.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Does Kinetic Energy Increase When Ice Melts As ice melts into water, kinetic energy is being added to the particles. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. At the melting point, they have. When the substance melts or boils, energy is put in to breaking the bonds that are holding particles together, which increases. Does Kinetic Energy Increase When Ice Melts.