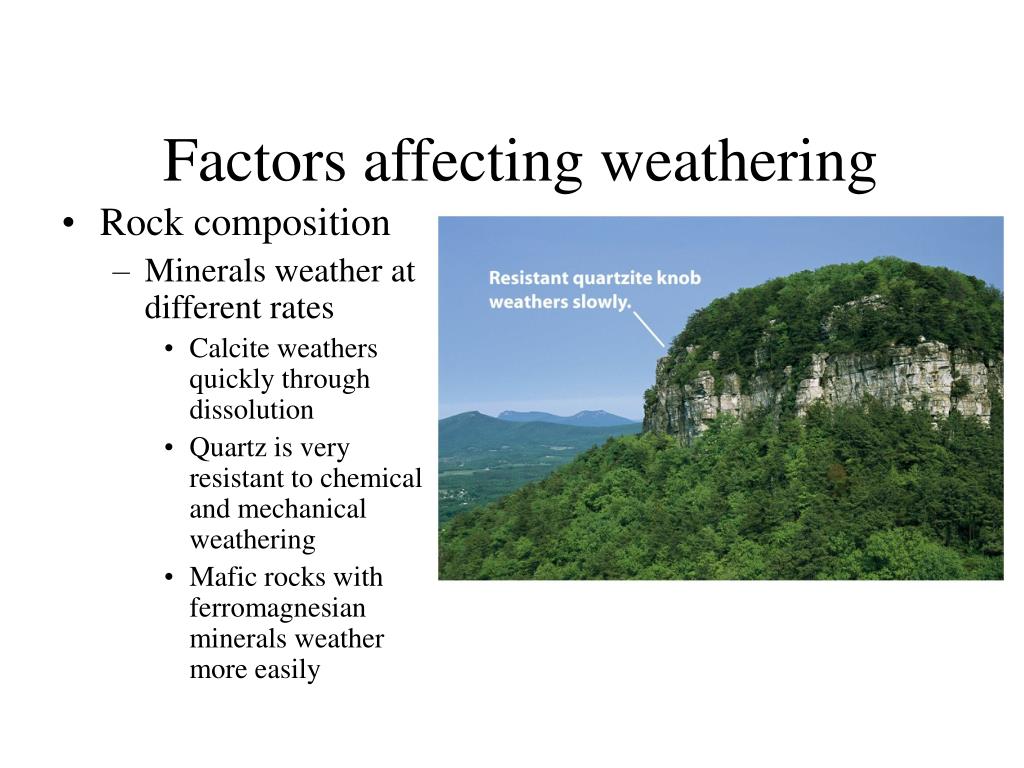
How Did Acid Precipitation Cause Rocks To Weather Faster . The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. There are two main types of weathering:.
from www.slideserve.com
Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. There are two main types of weathering:. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to.
PPT Chapter 7 WEATHERING AND EROSION PowerPoint Presentation, free
How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. There are two main types of weathering:. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to.
From bmxracingthailand.com
How Does Acid Precipitation Cause Rocks To Weather Faster? Update How Did Acid Precipitation Cause Rocks To Weather Faster The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slidemake.com
List Of Climate Tipping Points Presentation How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Weathering is the process of the weakening and breakdown of rocks, metals, and. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering and Soil Formation PowerPoint Presentation, free How Did Acid Precipitation Cause Rocks To Weather Faster Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The higher weathering rates. How Did Acid Precipitation Cause Rocks To Weather Faster.
From slideplayer.com
9.2 Rates Of Weathering. ppt download How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. There are two main types of weathering:. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The higher weathering rates in basic igneous rocks result in greater nutrient. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Chapter 7 WEATHERING AND EROSION PowerPoint Presentation, free How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Weathering is. How Did Acid Precipitation Cause Rocks To Weather Faster.
From slideplayer.com
Chapter 14 Weathering and Erosion ppt download How Did Acid Precipitation Cause Rocks To Weather Faster Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced. How Did Acid Precipitation Cause Rocks To Weather Faster.
From exohouuzn.blob.core.windows.net
Can Rain Water Cause Itching at Jacobs blog How Did Acid Precipitation Cause Rocks To Weather Faster There are two main types of weathering:. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Weathering is the process of the weakening and. How Did Acid Precipitation Cause Rocks To Weather Faster.
From owlcation.com
Chemical Weathering A Great Natural Force Owlcation How Did Acid Precipitation Cause Rocks To Weather Faster Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced. How Did Acid Precipitation Cause Rocks To Weather Faster.
From ar.inspiredpencil.com
Acid Precipitation Weathering Chamical How Did Acid Precipitation Cause Rocks To Weather Faster Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. There are two main types of weathering:. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared. How Did Acid Precipitation Cause Rocks To Weather Faster.
From geo.libretexts.org
8.7 Weathering and Erosion Geosciences LibreTexts How Did Acid Precipitation Cause Rocks To Weather Faster The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Dissolution can be enhanced by. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering and Soil Formation PowerPoint Presentation ID3032426 How Did Acid Precipitation Cause Rocks To Weather Faster The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.wdrb.com
What's the Difference Explaining Precipitation Types Weather Blog How Did Acid Precipitation Cause Rocks To Weather Faster Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Dissolution can be enhanced by. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.vecteezy.com
Diagram showing acid rain pathway on white background 1520150 Vector How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. There are two main types of weathering:. Acid rain,. How Did Acid Precipitation Cause Rocks To Weather Faster.
From rain-acid.weebly.com
Causes Acid Rain How Did Acid Precipitation Cause Rocks To Weather Faster There are two main types of weathering:. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids. How Did Acid Precipitation Cause Rocks To Weather Faster.
From ar.inspiredpencil.com
Acid Precipitation How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. There are two main types of weathering:. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The calcium carbonate (limestone). How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering and Soil Formation PowerPoint Presentation, free How Did Acid Precipitation Cause Rocks To Weather Faster Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Dissolution can be enhanced by a biological agent, such as when. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.worldatlas.com
How Do The Rocky Mountains Influence Climate? WorldAtlas How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. There are two main types of weathering:. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The higher weathering rates in basic igneous rocks. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.scienceshorts.com
What Does Acid Rain Do To Rocks How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.biologyexams4u.com
Biology Exams 4 U How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. There are two main types of weathering:. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial. How Did Acid Precipitation Cause Rocks To Weather Faster.
From ar.inspiredpencil.com
Acid Precipitation Cycle How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. There are two main types of weathering:. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Weathering is the process of the. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering and Soil Formation PowerPoint Presentation, free How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the. How Did Acid Precipitation Cause Rocks To Weather Faster.
From socratic.org
How does acid rain form? Socratic How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. The decaying remains of plants and some fungi form carbonic acid, which can weaken and.. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering and Soil Formation PowerPoint Presentation ID3032426 How Did Acid Precipitation Cause Rocks To Weather Faster Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Weathering is the process of the weakening and breakdown of. How Did Acid Precipitation Cause Rocks To Weather Faster.
From slideplayer.com
Weathering and Erosion ppt download How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything.. How Did Acid Precipitation Cause Rocks To Weather Faster.
From slideplayer.com
Unit 1 Lesson 2 Weathering ppt download How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The higher weathering rates. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas How Did Acid Precipitation Cause Rocks To Weather Faster Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the rocks they are attached to. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. There are two main. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Day one PowerPoint Presentation, free download ID1458696 How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. There are two main types of weathering:. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. The higher weathering rates in basic igneous rocks. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.mindomo.com
Environmental Issues Mind Map How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the. How Did Acid Precipitation Cause Rocks To Weather Faster.
From ar.inspiredpencil.com
Acid Precipitation Weathering Chamical How Did Acid Precipitation Cause Rocks To Weather Faster The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto the. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas How Did Acid Precipitation Cause Rocks To Weather Faster There are two main types of weathering:. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Dissolution can be enhanced by a biological agent, such as when organisms like lichen. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts How Did Acid Precipitation Cause Rocks To Weather Faster Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. There are two main types. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.slideserve.com
PPT Weathering & Soil Formation PowerPoint Presentation, free How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. Dissolution can be enhanced by a biological agent, such as when organisms like lichen and bacteria release organic acids onto. How Did Acid Precipitation Cause Rocks To Weather Faster.
From projectwed.weebly.com
Weathering WeD How Did Acid Precipitation Cause Rocks To Weather Faster The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. There are two main types of weathering:. The higher. How Did Acid Precipitation Cause Rocks To Weather Faster.
From www.researchgate.net
Sequential stages of rock weathering for soil formation and evolution How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. Weathering is the process of the weakening and breakdown of rocks, metals, and artificial objects. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. The calcium. How Did Acid Precipitation Cause Rocks To Weather Faster.
From ar.inspiredpencil.com
Acid Precipitation On Rocks How Did Acid Precipitation Cause Rocks To Weather Faster Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the. The decaying remains of plants and some fungi form carbonic acid, which can weaken and. The higher weathering rates in basic igneous rocks result in greater nutrient availability compared to acid igneous rocks, everything. There are. How Did Acid Precipitation Cause Rocks To Weather Faster.