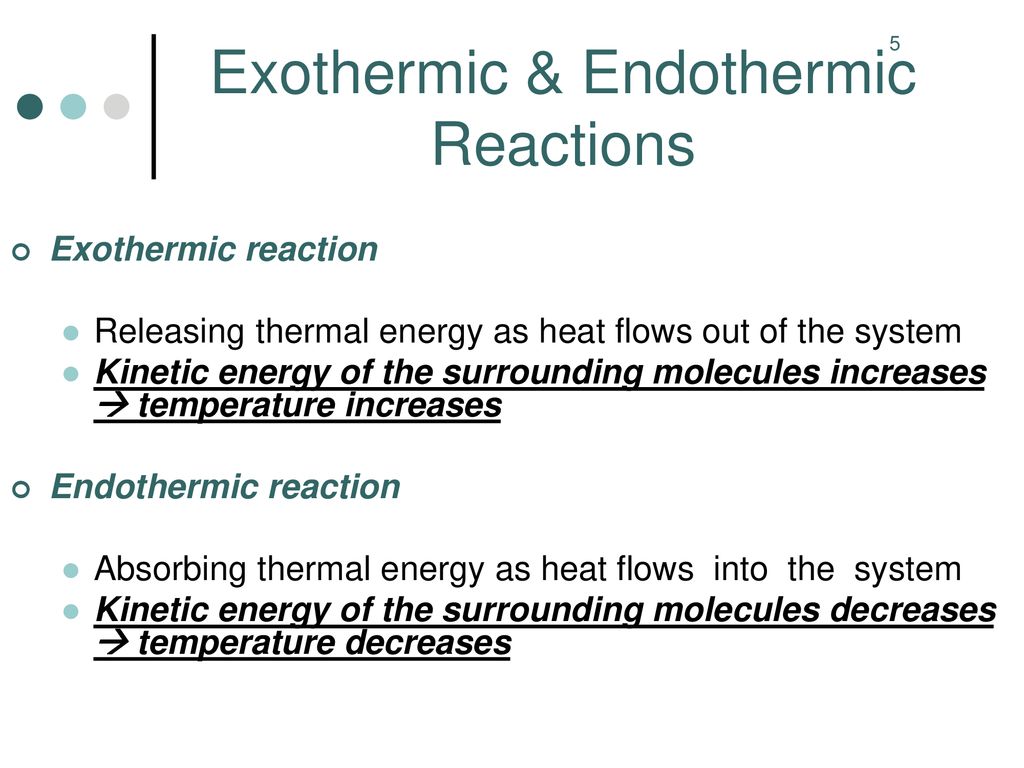
Endothermic Reaction Increase In Temperature . An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =. An endothermic chemical reaction causes a. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. A temperature change occurs when temperature is increased or decreased by the. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. An increase in temperature will. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Effect of temperature on equilibrium. Dissolution of ammonium nitrate in water, as in.
from slideplayer.com
When temperature is the stress that affects a system at equilibrium, there are two important consequences: An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Effect of temperature on equilibrium. A temperature change occurs when temperature is increased or decreased by the. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. An endothermic chemical reaction causes a. An increase in temperature will. Dissolution of ammonium nitrate in water, as in.
Thermochemistry Thermochemistry the study of the energy changes that physical or
Endothermic Reaction Increase In Temperature An increase in temperature will. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Effect of temperature on equilibrium. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. Dissolution of ammonium nitrate in water, as in. An endothermic chemical reaction causes a. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. When temperature is the stress that affects a system at equilibrium, there are two important consequences: A temperature change occurs when temperature is increased or decreased by the. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. An increase in temperature will. In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =.
From slideplayer.com
Reaction Rates and Le Chatelier’s Principle ppt download Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic chemical reaction causes a. When temperature is the stress that affects a system at equilibrium, there are two important consequences:. Endothermic Reaction Increase In Temperature.
From www.numerade.com
Is the solution process exothermic (energy released, temperature increases) or endothermic Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. An endothermic chemical reaction causes a. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In the second case, it makes sense that an endothermic reaction would favor an increase in temperature. Endothermic Reaction Increase In Temperature.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. When temperature is the stress that affects a system at equilibrium, there are two important consequences: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature will. By definition, an exothermic. Endothermic Reaction Increase In Temperature.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Increase In Temperature A temperature change occurs when temperature is increased or decreased by the. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Dissolution of ammonium nitrate in water, as in. An increase in temperature will. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An. Endothermic Reaction Increase In Temperature.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID6179063 Endothermic Reaction Increase In Temperature When temperature is the stress that affects a system at equilibrium, there are two important consequences: An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. Effect of temperature on equilibrium. In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =.. Endothermic Reaction Increase In Temperature.
From www.numerade.com
SOLVED In an endothermic reaction the heat capacity of the reactants is lower than the heat Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. A temperature change occurs when temperature is increased or decreased by the. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic. Endothermic Reaction Increase In Temperature.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Effect of temperature on equilibrium. An exothermic chemical reaction causes an increase in temperature (of the surroundings). A temperature change occurs when temperature is increased or decreased by the. An endothermic reaction occurs when the. Endothermic Reaction Increase In Temperature.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Increase In Temperature An increase in temperature will. Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an. Endothermic Reaction Increase In Temperature.
From www.slideshare.net
Gc chemical equilibrium Endothermic Reaction Increase In Temperature An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Dissolution of ammonium nitrate in water, as in. When temperature is the stress. Endothermic Reaction Increase In Temperature.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. When temperature is the stress that affects a system at equilibrium, there are two important consequences: In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =. An endothermic chemical reaction causes a. An endothermic reaction occurs when the temperature of an isolated. Endothermic Reaction Increase In Temperature.
From www.numerade.com
SOLVEDThe equilibrium constant for an endothermic reaction increases with rising temperature Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An endothermic chemical. Endothermic Reaction Increase In Temperature.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic reaction occurs when the temperature of an isolated system decreases. Endothermic Reaction Increase In Temperature.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic chemical reaction causes a. Effect of temperature on equilibrium. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. When. Endothermic Reaction Increase In Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Increase In Temperature When temperature is the stress that affects a system at equilibrium, there are two important consequences: Effect of temperature on equilibrium. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. A. Endothermic Reaction Increase In Temperature.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Increase In Temperature When temperature is the stress that affects a system at equilibrium, there are two important consequences: Effect of temperature on equilibrium. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. In the second. Endothermic Reaction Increase In Temperature.
From slideplayer.com
Thermochemistry Thermochemistry the study of the energy changes that physical or Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =. A temperature change occurs when temperature is increased or decreased by. Endothermic Reaction Increase In Temperature.
From www.numerade.com
SOLVED Consider the endothermic reaction 2 NHy(g) 2 3 H,(g) + N(g) . What effect does changes Endothermic Reaction Increase In Temperature An exothermic chemical reaction causes an increase in temperature (of the surroundings). When temperature is the stress that affects a system at equilibrium, there are two important consequences: An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. An endothermic chemical reaction causes a. Effect of temperature on equilibrium. Dissolution of. Endothermic Reaction Increase In Temperature.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). When temperature is the stress that affects a system at equilibrium, there are two important consequences: By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Effect of temperature on equilibrium. In an endothermic process, the heat that. Endothermic Reaction Increase In Temperature.
From www.bartleby.com
Answered For an endothermic reaction in… bartleby Endothermic Reaction Increase In Temperature A temperature change occurs when temperature is increased or decreased by the. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. Effect of temperature on equilibrium. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Dissolution of ammonium nitrate in water, as in.. Endothermic Reaction Increase In Temperature.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Effect of temperature on equilibrium. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). A temperature change occurs when temperature. Endothermic Reaction Increase In Temperature.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction Increase In Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. An endothermic chemical reaction causes a. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. A temperature change occurs when temperature is increased. Endothermic Reaction Increase In Temperature.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. A temperature change occurs when temperature is increased or decreased by the. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An increase in temperature will. In. Endothermic Reaction Increase In Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Increase In Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An increase in temperature leads to a greater frequency of. Endothermic Reaction Increase In Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Endothermic Reaction Increase In Temperature An exothermic chemical reaction causes an increase in temperature (of the surroundings). An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. Effect of temperature on equilibrium. Dissolution of ammonium nitrate in water, as in. In the second case, it makes sense that an endothermic reaction would favor an increase in. Endothermic Reaction Increase In Temperature.
From www.youtube.com
Le Chatelier's Principle and Temperature Changes (Pt. 10) YouTube Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When temperature is the stress that affects a system at equilibrium, there are two important consequences: An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. Endothermic Reaction Increase In Temperature.
From www.slideserve.com
PPT Section 8.4—Le Chatelier’s Principle PowerPoint Presentation ID6981845 Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. A temperature change occurs when temperature is increased or decreased by the. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. In an endothermic process, the heat that a system absorbs is. Endothermic Reaction Increase In Temperature.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Reaction Increase In Temperature An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur =. An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. An endothermic reaction occurs when the temperature of an. Endothermic Reaction Increase In Temperature.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Increase In Temperature An endothermic chemical reaction causes a. When temperature is the stress that affects a system at equilibrium, there are two important consequences: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In the second case, it makes sense that an. Endothermic Reaction Increase In Temperature.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Increase In Temperature An increase in temperature leads to a greater frequency of collisions between reactants and products, leading to an increased. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In an endothermic process, the heat that a system absorbs is. Endothermic Reaction Increase In Temperature.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Dissolution of ammonium nitrate in water, as in. An endothermic chemical reaction causes a. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g.. Endothermic Reaction Increase In Temperature.
From wisc.pb.unizin.org
Day 30 Le Châtelier’s Principle Chemistry 109 Endothermic Reaction Increase In Temperature An increase in temperature will. An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An endothermic chemical reaction causes a. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In the second case, it makes sense that an endothermic reaction would favor an increase in temperature because δssur. Endothermic Reaction Increase In Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Increase In Temperature When temperature is the stress that affects a system at equilibrium, there are two important consequences: An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. An increase in temperature will. Effect of temperature on equilibrium. A temperature change occurs when temperature is increased or decreased by the. An exothermic chemical reaction. Endothermic Reaction Increase In Temperature.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Increase In Temperature In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Effect of temperature on equilibrium. An increase in temperature will. An exothermic chemical reaction causes an increase in temperature (of the surroundings). By definition, an exothermic reaction. Endothermic Reaction Increase In Temperature.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Endothermic Reaction Increase In Temperature An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non. Effect of temperature on equilibrium. A temperature change occurs when temperature is increased or decreased by the. An endothermic chemical reaction causes a. Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the. Endothermic Reaction Increase In Temperature.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Increase In Temperature Dissolution of ammonium nitrate in water, as in. An exothermic chemical reaction causes an increase in temperature (of the surroundings). An increase in temperature will. When temperature is the stress that affects a system at equilibrium, there are two important consequences: A temperature change occurs when temperature is increased or decreased by the. An endothermic reaction occurs when the temperature. Endothermic Reaction Increase In Temperature.