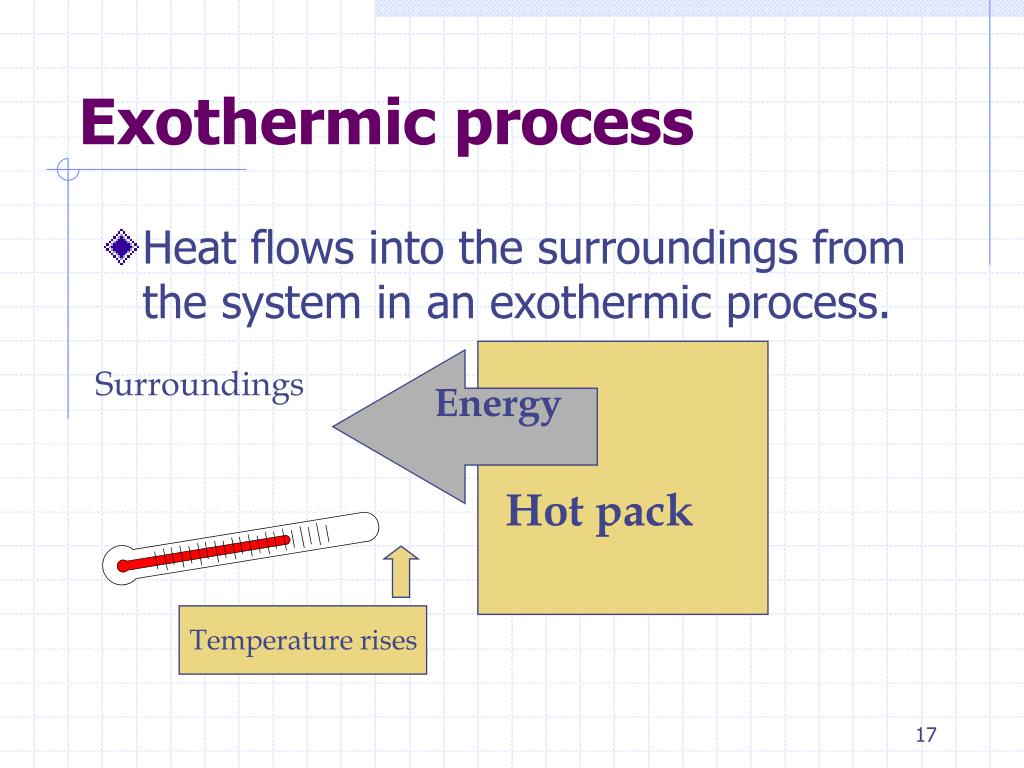
Is Using A Cold Pack Endothermic Or Exothermic . Are these reactions endothermic or exothermic? a cold pack used to treat muscle strains provides an example of an endothermic process. When the substances in the cold pack (water and a salt like. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. reactions that absorb heat from the environment are called endothermic reactions. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. A common example is a chemical ice pack,. instant cold packs use chemical reaction. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by.
from www.slideserve.com
Are these reactions endothermic or exothermic? green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. reactions that absorb heat from the environment are called endothermic reactions. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. instant cold packs use chemical reaction. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. A common example is a chemical ice pack,. When the substances in the cold pack (water and a salt like. a cold pack used to treat muscle strains provides an example of an endothermic process.
PPT Endothermic and exothermic reactions PowerPoint Presentation
Is Using A Cold Pack Endothermic Or Exothermic Are these reactions endothermic or exothermic? a cold pack used to treat muscle strains provides an example of an endothermic process. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. A common example is a chemical ice pack,. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Are these reactions endothermic or exothermic? instant cold packs use chemical reaction. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. reactions that absorb heat from the environment are called endothermic reactions. When the substances in the cold pack (water and a salt like.
From www.numerade.com
SOLVED 4. a) How does a cold pack work? Does it use an endothermic or Is Using A Cold Pack Endothermic Or Exothermic When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. A common example is a chemical ice pack,. reactions that absorb heat from the environment are called endothermic reactions. instant cold packs use chemical reaction. When the substances in the cold pack (water and a salt like. in. Is Using A Cold Pack Endothermic Or Exothermic.
From www.youtube.com
Make A Homemade Ice Pack Easy Endothermic Science Experiment YouTube Is Using A Cold Pack Endothermic Or Exothermic in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. When the substances in the cold pack (water and a salt like. a cold pack used to treat muscle strains provides an example of an endothermic process. reactions that absorb heat from the environment are. Is Using A Cold Pack Endothermic Or Exothermic.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Is Using A Cold Pack Endothermic Or Exothermic green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. Are these reactions endothermic or exothermic? in this lesson plan, students will explore several endothermic. Is Using A Cold Pack Endothermic Or Exothermic.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Is Using A Cold Pack Endothermic Or Exothermic instant cold packs use chemical reaction. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Are these reactions endothermic or exothermic? When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. A common example is a chemical ice pack,.. Is Using A Cold Pack Endothermic Or Exothermic.
From www.gauthmath.com
Label the following image of a cold pack to show the movement of Is Using A Cold Pack Endothermic Or Exothermic in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Are these reactions endothermic or exothermic? green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. When the cold pack is squeezed,. Is Using A Cold Pack Endothermic Or Exothermic.
From www.thoughtco.com
Create an Endothermic Chemical Reaction Is Using A Cold Pack Endothermic Or Exothermic these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. instant cold packs use chemical reaction. A common example is a chemical ice pack,. Are these reactions endothermic or exothermic? When the cold pack is squeezed,. Is Using A Cold Pack Endothermic Or Exothermic.
From schematicpaliza06.z22.web.core.windows.net
Exothermic And Endothermic Diagrams Is Using A Cold Pack Endothermic Or Exothermic Are these reactions endothermic or exothermic? A common example is a chemical ice pack,. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. instant cold packs use chemical reaction. reactions that absorb heat from the environment are called endothermic reactions. When the cold pack. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT CHEMISTRY 122 PowerPoint Presentation, free download ID6314666 Is Using A Cold Pack Endothermic Or Exothermic green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. a cold pack used to treat muscle strains provides an example of an endothermic process. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose. Is Using A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic and Exothermic Reactions ppt download Is Using A Cold Pack Endothermic Or Exothermic instant cold packs use chemical reaction. Are these reactions endothermic or exothermic? A common example is a chemical ice pack,. reactions that absorb heat from the environment are called endothermic reactions. When the substances in the cold pack (water and a salt like. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Using A Cold Pack Endothermic Or Exothermic green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h 2 o) by. instant cold packs use chemical reaction. Are these reactions endothermic or exothermic? A common example is a chemical ice pack,. a cold pack used to treat muscle strains provides an example of an. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Using A Cold Pack Endothermic Or Exothermic a cold pack used to treat muscle strains provides an example of an endothermic process. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. A common example is a chemical ice pack,. instant cold packs use chemical reaction. these cold packs have a strong outer plastic layer. Is Using A Cold Pack Endothermic Or Exothermic.
From courses.lumenlearning.com
The Dissolving Process CHEM 1305 Introductory Chemistry Is Using A Cold Pack Endothermic Or Exothermic these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. a cold pack used to treat muscle strains provides an example of an endothermic process. When the cold pack is squeezed, the inner bag of water. Is Using A Cold Pack Endothermic Or Exothermic.
From www.youtube.com
Instant hot pack and instant cold pack, application of exothermic and Is Using A Cold Pack Endothermic Or Exothermic Are these reactions endothermic or exothermic? these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. instant. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Using A Cold Pack Endothermic Or Exothermic A common example is a chemical ice pack,. When the substances in the cold pack (water and a salt like. a cold pack used to treat muscle strains provides an example of an endothermic process. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Using A Cold Pack Endothermic Or Exothermic reactions that absorb heat from the environment are called endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Are these reactions endothermic or exothermic? A common example is a chemical ice pack,. When the cold pack is squeezed, the inner bag of water breaks and the. Is Using A Cold Pack Endothermic Or Exothermic.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Is Using A Cold Pack Endothermic Or Exothermic instant cold packs use chemical reaction. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. A common example is a chemical ice pack,. When the substances in the cold pack (water and a salt like. green plants are capable of synthesizing glucose (c 6. Is Using A Cold Pack Endothermic Or Exothermic.
From brooklynewtortega.blogspot.com
Cold Pack Endothermic or Exothermic BrooklynewtOrtega Is Using A Cold Pack Endothermic Or Exothermic reactions that absorb heat from the environment are called endothermic reactions. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. a cold pack used to treat muscle strains provides an example of an endothermic. Is Using A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Is Using A Cold Pack Endothermic Or Exothermic A common example is a chemical ice pack,. When the substances in the cold pack (water and a salt like. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. a cold pack used to treat muscle strains provides an example of an endothermic process. . Is Using A Cold Pack Endothermic Or Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Is Using A Cold Pack Endothermic Or Exothermic a cold pack used to treat muscle strains provides an example of an endothermic process. Are these reactions endothermic or exothermic? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. in the course of an endothermic process, the system gains heat from the surroundings and so the. Is Using A Cold Pack Endothermic Or Exothermic.
From www.sciencebuddies.org
Cold Pack Chemistry Exploring Endothermic and Exothermic Reactions Is Using A Cold Pack Endothermic Or Exothermic When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. instant cold packs use chemical reaction. When the substances in the cold pack (water and a salt like. reactions that absorb heat from the environment are called endothermic reactions. in the course of an endothermic process, the system. Is Using A Cold Pack Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Is Using A Cold Pack Endothermic Or Exothermic reactions that absorb heat from the environment are called endothermic reactions. instant cold packs use chemical reaction. Are these reactions endothermic or exothermic? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. When the cold pack is squeezed, the inner bag of water breaks and the water. Is Using A Cold Pack Endothermic Or Exothermic.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Is Using A Cold Pack Endothermic Or Exothermic in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. reactions that absorb heat from the environment are called endothermic reactions. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. A common example is a chemical. Is Using A Cold Pack Endothermic Or Exothermic.
From www.youtube.com
Hot/Cold Pack Endothermic and Exothermic Reactions (4/11) YouTube Is Using A Cold Pack Endothermic Or Exothermic in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. reactions that absorb heat from the environment are called endothermic reactions. instant cold packs use chemical reaction. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download Is Using A Cold Pack Endothermic Or Exothermic When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. A common example is a chemical ice pack,. instant cold packs use chemical reaction. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. green plants are capable of. Is Using A Cold Pack Endothermic Or Exothermic.
From teachingscience.us
Exothermic and Endothermic Reactions Is Using A Cold Pack Endothermic Or Exothermic instant cold packs use chemical reaction. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. reactions that absorb heat from the environment are called endothermic reactions. green plants are capable of synthesizing glucose (c 6 h 12 o 6) from carbon dioxide (co 2) and water (h. Is Using A Cold Pack Endothermic Or Exothermic.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Is Using A Cold Pack Endothermic Or Exothermic Are these reactions endothermic or exothermic? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. reactions that absorb heat from the environment are called endothermic reactions. When the substances in the cold pack (water and a salt like. these cold packs have a strong outer plastic layer. Is Using A Cold Pack Endothermic Or Exothermic.
From www.thinkswap.com
Endothermic Reaction Practical (Science of Cold Packs) Chemistry Is Using A Cold Pack Endothermic Or Exothermic in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. reactions that absorb heat from the environment are called endothermic reactions. Are these reactions endothermic or exothermic? When the substances in the cold pack (water and a salt like. A common example is a chemical ice. Is Using A Cold Pack Endothermic Or Exothermic.
From physicsinmyview.com
10 Examples of Endothermic Reactions in Everyday Life Physics In My View Is Using A Cold Pack Endothermic Or Exothermic a cold pack used to treat muscle strains provides an example of an endothermic process. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Using A Cold Pack Endothermic Or Exothermic A common example is a chemical ice pack,. these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose. Is Using A Cold Pack Endothermic Or Exothermic.
From www.thoughtco.com
Endothermic Reaction Examples Is Using A Cold Pack Endothermic Or Exothermic When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. a cold pack used to treat muscle strains provides an example of an endothermic process. reactions that absorb heat from the environment are called endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions,. Is Using A Cold Pack Endothermic Or Exothermic.
From mylaldnorman.blogspot.com
Cold Pack Endothermic or Exothermic MylaldNorman Is Using A Cold Pack Endothermic Or Exothermic instant cold packs use chemical reaction. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Are these reactions endothermic or exothermic? a cold pack used to treat muscle strains provides an example of an endothermic process. reactions that absorb heat from the environment. Is Using A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Is Using A Cold Pack Endothermic Or Exothermic A common example is a chemical ice pack,. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Are these reactions endothermic or exothermic? instant cold packs use chemical reaction. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals.. Is Using A Cold Pack Endothermic Or Exothermic.
From www.joelgordon.com
Cold Pack an Endothermic Reaction Joel Gordon Photography Is Using A Cold Pack Endothermic Or Exothermic these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. reactions that absorb heat from the environment are called endothermic reactions. Are these reactions endothermic or exothermic? in the course of an endothermic process, the. Is Using A Cold Pack Endothermic Or Exothermic.
From www.pinterest.com
Cold Pack Chemistry Exploring Endothermic and Exothermic Reactions Is Using A Cold Pack Endothermic Or Exothermic in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. A common example is a chemical ice pack,. reactions that absorb heat from the environment are called endothermic reactions. When the cold pack is squeezed, the inner bag of water breaks and the water mixes with the chemicals. Are. Is Using A Cold Pack Endothermic Or Exothermic.
From learningschoolbocadeaswb.z4.web.core.windows.net
Endothermic Vs Exothermic Worksheet Is Using A Cold Pack Endothermic Or Exothermic these cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an endothermic reaction when dissolved in water. a cold pack used to treat muscle strains provides an example of an endothermic process. A common example is a chemical ice pack,. in this. Is Using A Cold Pack Endothermic Or Exothermic.