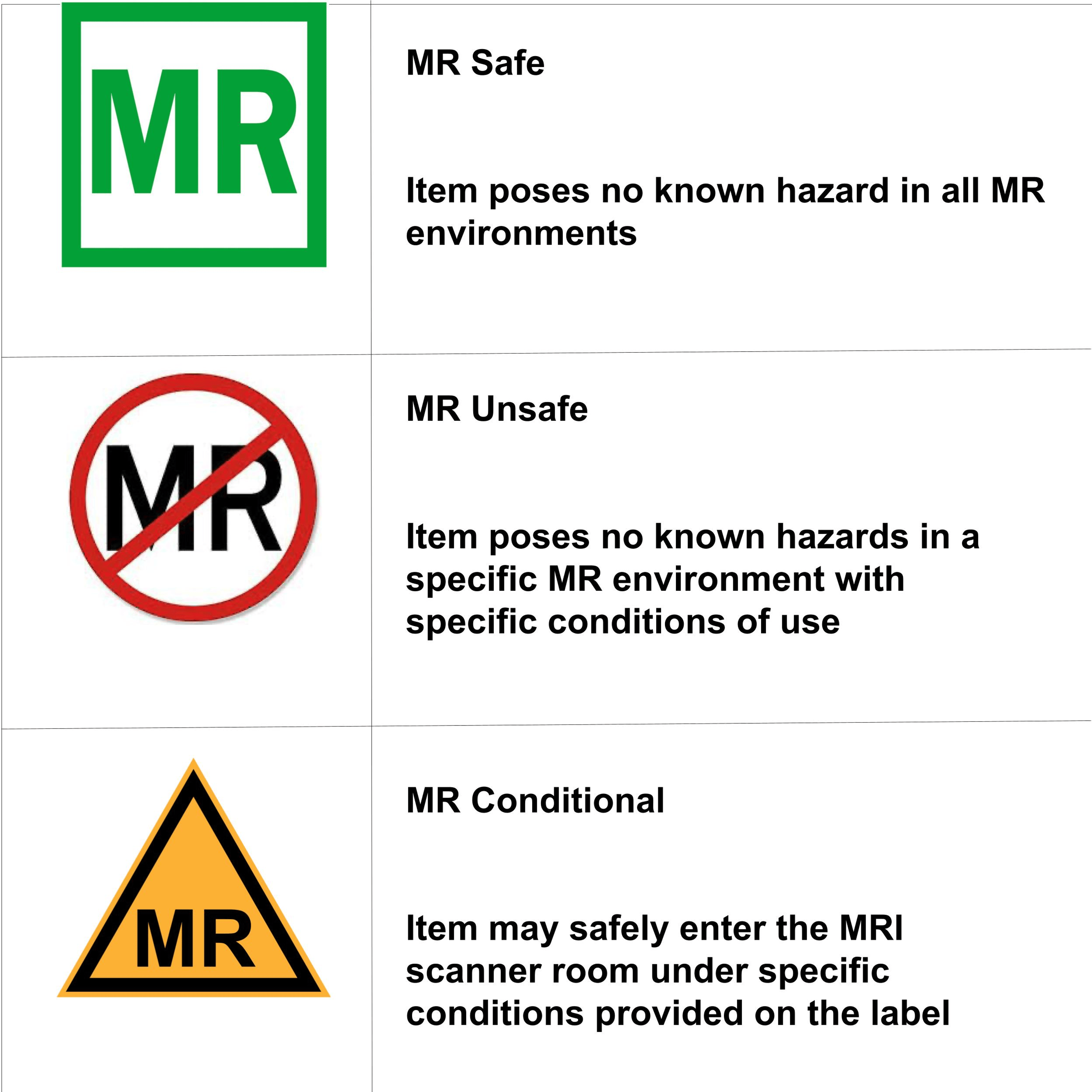
Mr Safe Labels . The acr safety committee thanks those in the mr. What condition(s) must be met to ensure the safety of the patient? Devices and implants can be mr safe, mr conditional or mr unsafe. The radiologist is about to put a patient with a synthes external fixation frame into the mri. In order to ensure patient. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Mri safety information and labeling. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. Acr guidance documents on mr safe practices, and the acr manual on mr safety.
from www.cureus.com
Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. Acr guidance documents on mr safe practices, and the acr manual on mr safety. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. What condition(s) must be met to ensure the safety of the patient? All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Devices and implants can be mr safe, mr conditional or mr unsafe. The acr safety committee thanks those in the mr. Mri safety information and labeling. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. The radiologist is about to put a patient with a synthes external fixation frame into the mri.
Cureus A Review of Resonance (MR) Safety The Essentials to
Mr Safe Labels Acr guidance documents on mr safe practices, and the acr manual on mr safety. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. Devices and implants can be mr safe, mr conditional or mr unsafe. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. What condition(s) must be met to ensure the safety of the patient? Mri safety information and labeling. Acr guidance documents on mr safe practices, and the acr manual on mr safety. The radiologist is about to put a patient with a synthes external fixation frame into the mri. In order to ensure patient. The acr safety committee thanks those in the mr.
From www.universalmedicalinc.com
MRI MRI Unsafe Stickers Mr Safe Labels The radiologist is about to put a patient with a synthes external fixation frame into the mri. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. Mri safety information and labeling. The. Mr Safe Labels.
From www.alamy.com
Bleach warning hires stock photography and images Alamy Mr Safe Labels Mri safety information and labeling. What condition(s) must be met to ensure the safety of the patient? The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Devices and implants can be mr safe, mr conditional or mr unsafe. The acr safety committee thanks those in the mr. The radiologist is. Mr Safe Labels.
From mrsafethailand.com
Mr.Safe Mr Safe Labels In order to ensure patient. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Devices. Mr Safe Labels.
From www.slideshare.net
Safety of MRI Critical Medical Devices Mr Safe Labels What condition(s) must be met to ensure the safety of the patient? In order to ensure patient. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The acr safety committee thanks those in. Mr Safe Labels.
From www.bamrr.org
Home British Association of MRI Radiographers Mr Safe Labels Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. In order to ensure patient. What condition(s) must be met to ensure the safety of the patient? The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Acr guidance documents on mr. Mr Safe Labels.
From www.universalmedicalinc.com
Z&Z Medical MR Conditional Sticker Mr Safe Labels This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. In order to ensure patient. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Acr guidance documents on mr safe practices, and the acr manual on mr safety. Terminology from the american. Mr Safe Labels.
From www.alimed.com
MRI Safe and Caution Stickers Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. In order to ensure patient. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration.. Mr Safe Labels.
From hnamedical.com
MRsafety signs, MRsafe/ conditional/ unsafe 2,5/5/8 cm (36 pc) H&A Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Devices and implants can be mr safe, mr conditional or mr unsafe. Acr guidance documents on mr safe practices, and the acr manual on mr safety. The acr safety committee thanks those in the mr. This guidance document provides the fda's. Mr Safe Labels.
From www.universalmedicalinc.com
Multi Pack of MR Safe MR Conditional MR Unsafe Stickers Mr Safe Labels Mri safety information and labeling. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Devices and implants can be mr safe, mr conditional or mr unsafe. In order to ensure patient. This. Mr Safe Labels.
From bigamart.com
10Pack MR Safe Label MRI Safe Sticker for Radiology 4 x 4 inch Mr Safe Labels Mri safety information and labeling. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Acr guidance documents on mr safe practices, and the acr manual on mr safety. What condition(s) must be met to ensure the safety of the patient? In order to ensure patient. Terminology from the american society for. Mr Safe Labels.
From www.universalmedicalinc.com
MRI MRI Safe Stickers Mr Safe Labels The radiologist is about to put a patient with a synthes external fixation frame into the mri. Acr guidance documents on mr safe practices, and the acr manual on mr safety. Devices and implants can be mr safe, mr conditional or mr unsafe. What condition(s) must be met to ensure the safety of the patient? Terminology from the american society. Mr Safe Labels.
From www.mrsafety.ind.br
MR Safety Indústria Mr Safe Labels In order to ensure patient. What condition(s) must be met to ensure the safety of the patient? Mri safety information and labeling. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. The. Mr Safe Labels.
From neuronewsinternational.com
MRI conditionality across spinal cord stimulation devices the myths Mr Safe Labels The acr safety committee thanks those in the mr. Acr guidance documents on mr safe practices, and the acr manual on mr safety. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Devices and implants can be mr safe, mr conditional or mr unsafe. Terminology from the american society for testing and. Mr Safe Labels.
From www.walmart.com
Safety Data Sheet Stickers 1.5" x 2.5", 2 Rolls of 250, Right to Know Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. In order to ensure patient. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. What condition(s) must be met to ensure the safety of the patient? Devices and implants can be mr. Mr Safe Labels.
From lemproductsinc.com
Transformer Label Mr. Ouch WARNING HAZARDOUS VOLTAGE, 4.5" X 8.5" LEM Mr Safe Labels Acr guidance documents on mr safe practices, and the acr manual on mr safety. Devices and implants can be mr safe, mr conditional or mr unsafe. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The radiologist is about to put a patient with a synthes external fixation frame into. Mr Safe Labels.
From www.grainger.com
GHS SAFETY Poster, Chemical Safety, English 10X329GHS1010 Grainger Mr Safe Labels Devices and implants can be mr safe, mr conditional or mr unsafe. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. In order to ensure patient. What condition(s) must be met to ensure the safety of the patient? The radiologist is about to put a patient with a synthes external. Mr Safe Labels.
From www.bir.org.uk
MR Safety British Institute of Radiology Mr Safe Labels The radiologist is about to put a patient with a synthes external fixation frame into the mri. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. What condition(s) must be met to. Mr Safe Labels.
From www.desertcart.ae
Buy MRI Warning Stickers 2X2 Inch MR Safe MRI Safe Vinyl Sticker MR Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. The acr safety committee thanks those in the mr. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. Acr guidance documents on mr safe practices, and the acr manual on mr safety.. Mr Safe Labels.
From www.regdesk.co
FDA Revised Guidance on MR Safety Displacement Force and Torque RegDesk Mr Safe Labels This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. What condition(s) must be met. Mr Safe Labels.
From www.cureus.com
Cureus A Review of Resonance (MR) Safety The Essentials to Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Devices and implants can be mr safe, mr conditional or mr unsafe. Acr guidance documents on mr safe practices, and the acr manual on mr safety. In order to ensure patient. All medical device labels are to include the name and. Mr Safe Labels.
From www.ohsu.edu
MRI Safety OHSU Mr Safe Labels Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The radiologist is about to put a patient with a synthes external fixation frame into the mri. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. The acr safety committee thanks those. Mr Safe Labels.
From www.mrsafetydrivingschool.com
Home Mr. Safety Driving Mr Safe Labels The acr safety committee thanks those in the mr. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Devices and implants can be mr safe, mr conditional or mr unsafe. What condition(s) must be met to ensure the safety of the patient? Terminology from the american society for testing and. Mr Safe Labels.
From magmedix.com
MRI Zone Sign Set Mr Safe Labels The radiologist is about to put a patient with a synthes external fixation frame into the mri. The acr safety committee thanks those in the mr. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. All medical device labels are to include the name and address of the manufacturer, packer,. Mr Safe Labels.
From www.sciencephoto.com
Drain cleaner hazard warning notices Stock Image C004/7697 Mr Safe Labels What condition(s) must be met to ensure the safety of the patient? Acr guidance documents on mr safe practices, and the acr manual on mr safety. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of. Mr Safe Labels.
From www.alimed.com
MRI Safe and Caution Stickers Mr Safe Labels Devices and implants can be mr safe, mr conditional or mr unsafe. Acr guidance documents on mr safe practices, and the acr manual on mr safety. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Terminology from the american society for testing and materials (astm) international and utilized by the food and. Mr Safe Labels.
From www.medicalimagingsource.com
Best MRI Safety Products Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. The radiologist is about to put a patient with a synthes external fixation frame into the mri. Acr guidance documents on mr safe practices, and the acr manual on mr safety. Mri safety information and labeling. In order to ensure patient.. Mr Safe Labels.
From www.canadiantire.ca
Mr. Clean Disinfectant MultiSurface Cleaner, Summer Citrus, 3.78L Mr Safe Labels Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The radiologist is about to put a patient with a synthes external fixation frame into the mri. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. In order to ensure patient. Mri. Mr Safe Labels.
From www.iis.fraunhofer.de
MR Safety Mr Safe Labels Acr guidance documents on mr safe practices, and the acr manual on mr safety. In order to ensure patient. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. The acr safety committee. Mr Safe Labels.
From hnamedical.com
MRsafety signs, MRsafe/ conditional/ unsafe 2,5/5/8 cm (36 pc) H&A Mr Safe Labels Mri safety information and labeling. In order to ensure patient. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not. Mr Safe Labels.
From mavink.com
Transformer Warning Sign Mr Safe Labels The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. Acr guidance documents on mr safe practices, and the acr manual on mr safety. Mri safety information and labeling. All medical device labels. Mr Safe Labels.
From wardray-premise.com
MR ASTM Labels Mr Safe Labels Devices and implants can be mr safe, mr conditional or mr unsafe. This guidance document provides the fda's recommendations on testing to assess the safety and compatibility of medical devices. Acr guidance documents on mr safe practices, and the acr manual on mr safety. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr. Mr Safe Labels.
From www.apsf.org
Airway Emergencies and Safety in Resonance Imaging (MRI) Suite Mr Safe Labels In order to ensure patient. What condition(s) must be met to ensure the safety of the patient? Acr guidance documents on mr safe practices, and the acr manual on mr safety. Devices and implants can be mr safe, mr conditional or mr unsafe. Mri safety information and labeling. The acr safety committee thanks those in the mr. Terminology from the. Mr Safe Labels.
From my.mistersafetyshoes.com
Mister Safety Shoes Inc Homepage Mr Safe Labels All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. Devices and implants can. Mr Safe Labels.
From www.universalmedicalinc.com
MRI MRI Safe Stickers Mr Safe Labels Devices and implants can be mr safe, mr conditional or mr unsafe. The acr's mr safety recommendations evidently apply to conventional clinical mr systems, not to specialized mr systems (e.g., dedicated. Mri safety information and labeling. All medical device labels are to include the name and address of the manufacturer, packer, or distributor, along. Terminology from the american society for. Mr Safe Labels.
From www.alimed.com
MRI Safe and Caution Stickers Mr Safe Labels Mri safety information and labeling. Acr guidance documents on mr safe practices, and the acr manual on mr safety. Devices and implants can be mr safe, mr conditional or mr unsafe. Terminology from the american society for testing and materials (astm) international and utilized by the food and drug administration. The acr safety committee thanks those in the mr. In. Mr Safe Labels.