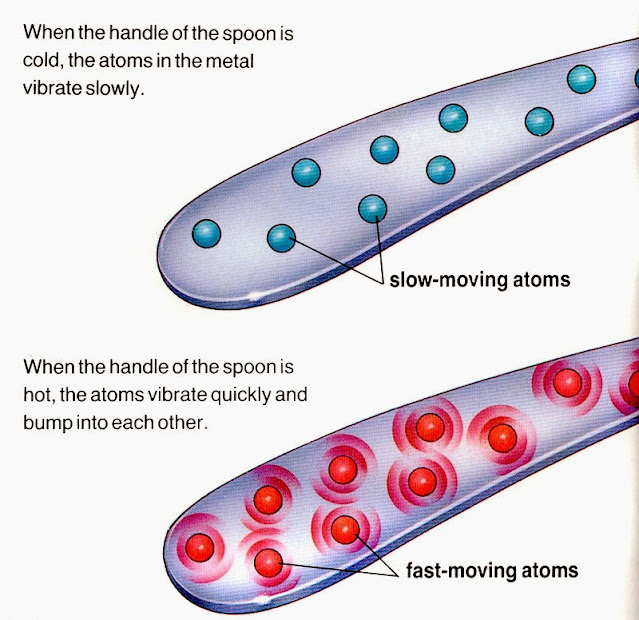
A R E Metals Good Conductors . That is why the parts of electrical objects that need to let electricity. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Many metals, such as copper, iron and steel, are good electrical conductors. But why are they, such good conductors? Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. They are good conductors of thermal energy because their delocalised electrons transfer energy. The electrons move from the negative terminal to the positive terminal. Melting point the temperature at which. Metals are excellent conductors of both heat and electricity.
from www.ency123.com
The electrons move from the negative terminal to the positive terminal. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. They are good conductors of thermal energy because their delocalised electrons transfer energy. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. But why are they, such good conductors? That is why the parts of electrical objects that need to let electricity. Metals are excellent conductors of both heat and electricity.
Why are metals good conductors of heat and electricity?
A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Melting point the temperature at which. The electrons move from the negative terminal to the positive terminal. That is why the parts of electrical objects that need to let electricity. Metals are excellent conductors of both heat and electricity. Many metals, such as copper, iron and steel, are good electrical conductors. But why are they, such good conductors? They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good.
From slideplayer.com
Chapter 6 Chemical Bonds ppt download A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. That is why the parts of electrical objects that need to let electricity. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Many metals, such as copper, iron and steel, are good. A R E Metals Good Conductors.
From www.youtube.com
Why are metals good conductors of electricity? \( \mathrm{PW} \) YouTube A R E Metals Good Conductors That is why the parts of electrical objects that need to let electricity. Metals are excellent conductors of both heat and electricity. The electrons move from the negative terminal to the positive terminal. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are good conductors of electricity because their delocalised electrons can carry a charge. A R E Metals Good Conductors.
From www.youtube.com
4.5 Why are Metals Malleable and Good Conductors? [SL IB Chemistry A R E Metals Good Conductors But why are they, such good conductors? Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. The electrons move from the negative terminal to the positive terminal. Many metals, such as copper,. A R E Metals Good Conductors.
From www.scienceabc.com
Why Are Metals Good Conductors Of Electricity And Heat? A R E Metals Good Conductors Metals are excellent conductors of both heat and electricity. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Many metals, such as copper, iron and steel, are good electrical conductors. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. That. A R E Metals Good Conductors.
From www.slideserve.com
PPT Lecture VII PowerPoint Presentation, free download ID1586922 A R E Metals Good Conductors That is why the parts of electrical objects that need to let electricity. Many metals, such as copper, iron and steel, are good electrical conductors. Melting point the temperature at which. The electrons move from the negative terminal to the positive terminal. But why are they, such good conductors? Metals are good conductors of electricity because their delocalised electrons can. A R E Metals Good Conductors.
From www.ency123.com
Why are metals good conductors of heat and electricity? A R E Metals Good Conductors Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. That is why the parts of electrical objects that need to let electricity. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Metals are excellent conductors of both heat and electricity. But. A R E Metals Good Conductors.
From lessonberginthurston.z21.web.core.windows.net
Copper Is A Good Conductor A R E Metals Good Conductors But why are they, such good conductors? Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Metals are excellent conductors of both heat and electricity. That is why the parts of electrical objects that need to let electricity. Many metals, such as copper, iron and steel, are good electrical. A R E Metals Good Conductors.
From www.slideshare.net
Chapter 24 Conduction A R E Metals Good Conductors Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. They are good conductors of thermal energy because their delocalised electrons transfer energy. That is why the parts of electrical objects that need to let electricity. They are used in industry and domestically for applications that require both thermal transfer and electrical energy. A R E Metals Good Conductors.
From www.youtube.com
To Show that Metals are Good Conductors of Heat and Have High Melting A R E Metals Good Conductors They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are excellent conductors of both heat and electricity. The electrons move from the negative terminal to the positive terminal. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. But why are they,. A R E Metals Good Conductors.
From slideplayer.com
KS3 Physics 8I Heating and Cooling. ppt download A R E Metals Good Conductors That is why the parts of electrical objects that need to let electricity. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. But why are they, such good conductors? The electrons move from the negative terminal to the positive terminal. Metals are excellent conductors of both heat and electricity. They are good. A R E Metals Good Conductors.
From www.thoughtco.com
Electrical Conductivity of Metals A R E Metals Good Conductors Metals are excellent conductors of both heat and electricity. That is why the parts of electrical objects that need to let electricity. Many metals, such as copper, iron and steel, are good electrical conductors. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. They are used in industry and domestically for applications. A R E Metals Good Conductors.
From thevigyan.com
Why Are Metals Good Conductors of Heat? The Vigyan A R E Metals Good Conductors Metals are excellent conductors of both heat and electricity. The electrons move from the negative terminal to the positive terminal. They are good conductors of thermal energy because their delocalised electrons transfer energy. But why are they, such good conductors? Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Many metals, such. A R E Metals Good Conductors.
From advayaameera.blogspot.com
28+ Why Are Metals Good Conductors AdvayAameera A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. But why are they, such good conductors? That is why the parts of electrical objects that need to let electricity. Many metals, such as copper, iron and steel, are good electrical conductors. Metals are excellent conductors of both heat and. A R E Metals Good Conductors.
From telgurus.co.uk
Why are metals good conductors of electricity? Science Questionnaire A R E Metals Good Conductors Melting point the temperature at which. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. But why are they, such good conductors? Many metals, such as copper, iron and steel, are good. A R E Metals Good Conductors.
From www.youtube.com
Why metals are good conductors of heat ? YouTube A R E Metals Good Conductors But why are they, such good conductors? Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. That is why the parts of electrical objects that need to let electricity. The electrons move. A R E Metals Good Conductors.
From engineerfix.com
The Reasons Why Metals Are Such Good Conductors Engineer Fix A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. They are good conductors of thermal energy because their delocalised electrons transfer energy. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are excellent conductors of both heat and electricity.. A R E Metals Good Conductors.
From illuminateelectric.blogspot.com
11+ Metals Are Good Conductors Of Electricity Because They For You A R E Metals Good Conductors The electrons move from the negative terminal to the positive terminal. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. But. A R E Metals Good Conductors.
From slideplayer.com
Organization of the Periodic Table ppt download A R E Metals Good Conductors That is why the parts of electrical objects that need to let electricity. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. The electrons move from the negative terminal to the positive terminal. They are good conductors of thermal energy because their delocalised electrons transfer energy. They are used in industry and. A R E Metals Good Conductors.
From blog.stuidapp.com
Everything you need to know about Conductors A R E Metals Good Conductors Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. They are good conductors of thermal energy because their delocalised electrons transfer energy. Melting point the temperature at which. That is why the. A R E Metals Good Conductors.
From www.youtube.com
Metals Are Good Conductors Of Electricity Diagram / How To Draw A R E Metals Good Conductors Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Metals are excellent conductors of both heat and electricity. But why are they, such good conductors? Melting point the temperature at which. They are good conductors of thermal energy because their delocalised electrons transfer energy. That is why the parts of electrical objects. A R E Metals Good Conductors.
From learningmole.com
Are Metals Good Conductors? LearningMole A R E Metals Good Conductors Melting point the temperature at which. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. But why are they, such good conductors? That is why the parts of electrical objects that need to let electricity. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the. A R E Metals Good Conductors.
From www.gkseries.com
Metals are good conductors of heat because A R E Metals Good Conductors The electrons move from the negative terminal to the positive terminal. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Melting point the temperature at which. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are good conductors of electricity because their delocalised electrons can carry. A R E Metals Good Conductors.
From advayaameera.blogspot.com
28+ Why Are Metals Good Conductors AdvayAameera A R E Metals Good Conductors They are good conductors of thermal energy because their delocalised electrons transfer energy. The electrons move from the negative terminal to the positive terminal. Metals are excellent conductors of both heat and electricity. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are good conductors of electricity because their delocalised. A R E Metals Good Conductors.
From www.ehow.com
What Metals Make Good Conductors of Electricity? Sciencing A R E Metals Good Conductors Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. The electrons move from the negative terminal to the positive terminal. But why are they, such good conductors? Metals are excellent conductors of both heat. A R E Metals Good Conductors.
From aracely-has-beard.blogspot.com
Why Are Metals Good Conductors of Both Heat and Electricity Aracely A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. Melting point the temperature at which. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the. A R E Metals Good Conductors.
From www.slideserve.com
PPT Electrical Electrical Conductors & Insulators PowerPoint A R E Metals Good Conductors Metals are excellent conductors of both heat and electricity. But why are they, such good conductors? That is why the parts of electrical objects that need to let electricity. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. They are good conductors of thermal energy because their delocalised electrons transfer energy.. A R E Metals Good Conductors.
From goldleafdesign4134.blogspot.com
Is Gold A Conductor Of Electricity / Is Gold Is A Good Conductor Of A R E Metals Good Conductors Melting point the temperature at which. But why are they, such good conductors? Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are excellent conductors of both heat and electricity. That is why the parts of electrical objects. A R E Metals Good Conductors.
From www.youtube.com
Materials that are Good Conductor of Heat and Electricity YouTube A R E Metals Good Conductors They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are excellent conductors of both heat and electricity. Many metals, such as copper, iron and steel, are good electrical conductors. Melting point the temperature at which. But why are they, such good conductors? Metals are good conductors of electricity because their. A R E Metals Good Conductors.
From www.vrogue.co
The Five Electrical Conductors And Their Uses vrogue.co A R E Metals Good Conductors Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. But why are they, such good conductors? Melting point the temperature at which. That is why the parts of electrical objects that need to let electricity. Metals are excellent conductors of both heat and electricity. They are used in industry. A R E Metals Good Conductors.
From www.slideserve.com
PPT Physical Properties PowerPoint Presentation, free download ID A R E Metals Good Conductors But why are they, such good conductors? They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are excellent conductors of both heat and electricity. Melting point the temperature at which. That is why the parts of electrical objects that need to let electricity. Materials that allow the flow of charge (current) or thermal energy (heat). A R E Metals Good Conductors.
From readership.org
Why Are Metals Good Conductors Of Both Heat And Electricity? Exploring A R E Metals Good Conductors Melting point the temperature at which. That is why the parts of electrical objects that need to let electricity. But why are they, such good conductors? The electrons move from the negative terminal to the positive terminal. They are good conductors of thermal energy because their delocalised electrons transfer energy. Metals are good conductors of electricity because their delocalised electrons. A R E Metals Good Conductors.
From www.plcgurus.net
Why Are Metals Good Conductors of Electricity? A Closer Look A R E Metals Good Conductors Many metals, such as copper, iron and steel, are good electrical conductors. That is why the parts of electrical objects that need to let electricity. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. They are good conductors of thermal energy because their delocalised electrons transfer energy. They are used in industry. A R E Metals Good Conductors.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity A R E Metals Good Conductors Many metals, such as copper, iron and steel, are good electrical conductors. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Metals are good conductors of electricity because their delocalised electrons can carry a charge within the structure. Melting point the temperature at which. Materials that allow the flow of charge. A R E Metals Good Conductors.
From www.sequoia-brass-copper.com
Which Metals Conduct Electricity? A R E Metals Good Conductors The electrons move from the negative terminal to the positive terminal. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. Materials that allow the flow of charge (current) or thermal energy (heat) through it with less resistance are considered good. But why are they, such good conductors? That is why the. A R E Metals Good Conductors.
From maisiewood.z13.web.core.windows.net
Conductivity Chart Of Metal A R E Metals Good Conductors The electrons move from the negative terminal to the positive terminal. Metals are excellent conductors of both heat and electricity. Melting point the temperature at which. Many metals, such as copper, iron and steel, are good electrical conductors. They are used in industry and domestically for applications that require both thermal transfer and electrical energy transfer. Materials that allow the. A R E Metals Good Conductors.