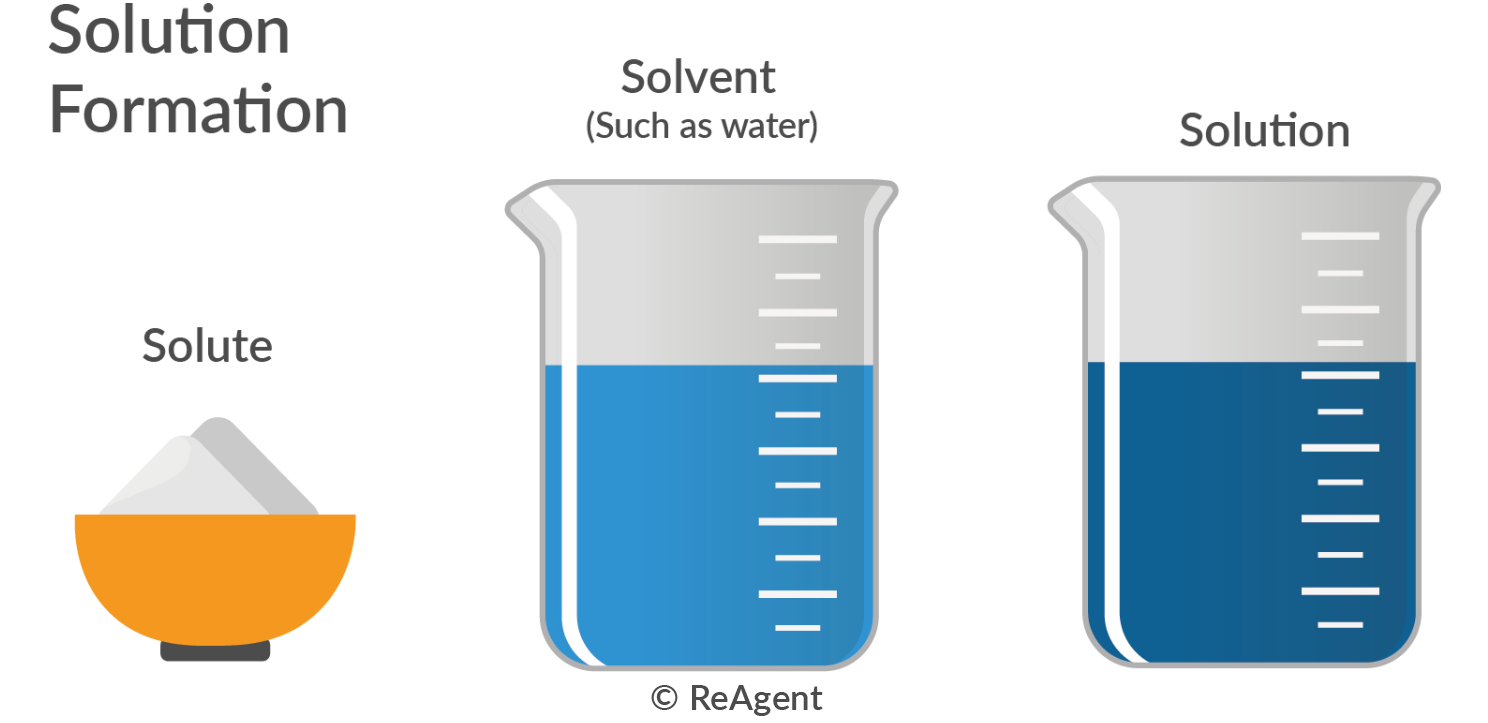
Primary Standard Vs Standard Solution . A standard solution can be prepared directly from a primary standard. Primary standards and secondary standards. It must also have a high level of purity, stability, and solubility in water. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. High state of purity, stability in air and in. A primary standard solution is a solution with a high purity and less reactivity. A solution of accurately known concentration, prepared using standard substances in one of several ways. A primary standard must have, at least, the following characteristics: Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. A primary standard is a reagent that we can use to dispense an accurately known. A secondary standard is not that pure and is chemically reactive than primary standards. There are two categories of analytical standards: It typically has a relatively high molar mass that can be accurately measured for comparison. A standard solution is measured.
from www.chemicals.co.uk
It typically has a relatively high molar mass that can be accurately measured for comparison. Primary standards and secondary standards. High state of purity, stability in air and in. A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard is a reagent that we can use to dispense an accurately known. A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. A standard solution can be prepared directly from a primary standard. There are two categories of analytical standards: A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution.
What Is A Standard Solution? The Chemistry Blog
Primary Standard Vs Standard Solution Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. High state of purity, stability in air and in. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard must have, at least, the following characteristics: A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard solution is a solution with a high purity and less reactivity. Primary standards and secondary standards. A solution of accurately known concentration, prepared using standard substances in one of several ways. It typically has a relatively high molar mass that can be accurately measured for comparison. A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. There are two categories of analytical standards: It must also have a high level of purity, stability, and solubility in water. A standard solution can be prepared directly from a primary standard. A standard solution is measured.
From www.slideserve.com
PPT Volumetric Analysis AcidBase PowerPoint Presentation, free Primary Standard Vs Standard Solution Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. It typically has a relatively high molar mass that can be accurately measured for comparison. Primary standards and secondary standards. A secondary standard is not that pure and is chemically. Primary Standard Vs Standard Solution.
From www.coursehero.com
[Solved] Can you please explain Why primary and secondary standard Primary Standard Vs Standard Solution A standard solution is measured. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard must have, at least, the following characteristics: A standard solution can be prepared directly from a primary standard. A secondary standard is not that pure and is chemically reactive than primary standards. A standard solution is any. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Primary Standard Vs Standard Solution A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard solution is a solution with a high purity and less reactivity. A solution of accurately known concentration, prepared using standard substances in one of several ways. A standard solution can be prepared directly from a primary standard. Primary standards and secondary standards. A. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry / PHC 213 PowerPoint Primary Standard Vs Standard Solution A secondary standard is not that pure and is chemically reactive than primary standards. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. It typically has a relatively high molar mass that can be accurately measured for comparison. A. Primary Standard Vs Standard Solution.
From pediaa.com
Difference Between Primary and Secondary Standard Solution Definition Primary Standard Vs Standard Solution A primary standard is a reagent that we can use to dispense an accurately known. A secondary standard is not that pure and is chemically reactive than primary standards. Primary standards and secondary standards. A solution of accurately known concentration, prepared using standard substances in one of several ways. A standard solution can be prepared directly from a primary standard.. Primary Standard Vs Standard Solution.
From www.youtube.com
difference between primary and secondary standard solution YouTube Primary Standard Vs Standard Solution A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. High state of purity, stability in air and in. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard must have, at least, the following characteristics: A standard solution is measured. A. Primary Standard Vs Standard Solution.
From www.differencebetween.com
Difference Between Stock Solution and Standard Solution Compare the Primary Standard Vs Standard Solution It typically has a relatively high molar mass that can be accurately measured for comparison. A primary standard solution is a solution with a high purity and less reactivity. High state of purity, stability in air and in. A primary standard must have, at least, the following characteristics: A primary standard is a reagent that we can use to dispense. Primary Standard Vs Standard Solution.
From www.studocu.com
Primary Standard PRIMARY STANDARD RECAP A primary standard solution Primary Standard Vs Standard Solution A solution of accurately known concentration, prepared using standard substances in one of several ways. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard solution is a solution with a high. Primary Standard Vs Standard Solution.
From www.bharatagritech.com
What Is A Primary Standard In Chemistry?, 47 OFF Primary Standard Vs Standard Solution A primary standard is a reagent that we can use to dispense an accurately known. A secondary standard is not that pure and is chemically reactive than primary standards. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. A. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry / PHC 213 PowerPoint Primary Standard Vs Standard Solution A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A solution of accurately known concentration, prepared using standard substances in one of several ways. A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. A primary standard must have,. Primary Standard Vs Standard Solution.
From www.youtube.com
What is Standard Solution Primary standard and Secondary Standard Primary Standard Vs Standard Solution It typically has a relatively high molar mass that can be accurately measured for comparison. A standard solution can be prepared directly from a primary standard. A primary standard is a reagent that we can use to dispense an accurately known. A solution of accurately known concentration, prepared using standard substances in one of several ways. High state of purity,. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT CHAPTER 6 TITRIMETRIC METHODS OF ANALYSIS PowerPoint Presentation Primary Standard Vs Standard Solution A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard solution is a solution with a high purity and less reactivity. A primary standard is a reagent that we can use to dispense an accurately known. A solution of accurately known concentration, prepared using standard substances in one of several ways. A standard. Primary Standard Vs Standard Solution.
From www.nagwa.com
Question Video Recalling the Meaning of the Term Standard Solution Nagwa Primary Standard Vs Standard Solution It must also have a high level of purity, stability, and solubility in water. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. A standard solution can be prepared directly from a primary standard. There are two categories of. Primary Standard Vs Standard Solution.
From www.youtube.com
Standard Solution Primary Standard Secondary Standard Analytical Primary Standard Vs Standard Solution A solution of accurately known concentration, prepared using standard substances in one of several ways. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. It must also have a high level of purity, stability, and solubility in water. A primary standard must have, at least, the following characteristics: Reagents. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Primary Standard Vs Standard Solution High state of purity, stability in air and in. Primary standards and secondary standards. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. There are two categories of analytical standards: A solution of accurately known concentration, prepared using standard substances in one of several ways. It typically has a. Primary Standard Vs Standard Solution.
From brainly.com
the difference between primary ans secondary standard solution Primary Standard Vs Standard Solution A primary standard is a reagent that we can use to dispense an accurately known. A standard solution is measured. High state of purity, stability in air and in. A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles. Primary Standard Vs Standard Solution.
From www.youtube.com
Primary Standard Solutions YouTube Primary Standard Vs Standard Solution Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. High state of purity, stability in air and in. A primary standard must have, at least, the following characteristics: A primary standard is a reagent that we can use to. Primary Standard Vs Standard Solution.
From www.chemicals.co.uk
Examples of Standard Solutions The Chemistry Blog Primary Standard Vs Standard Solution It typically has a relatively high molar mass that can be accurately measured for comparison. Primary standards and secondary standards. A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. A primary standard solution is a solution with a high purity and less reactivity. A standard solution is measured. A. Primary Standard Vs Standard Solution.
From docslib.org
Primary Standard and Secondary Standard DocsLib Primary Standard Vs Standard Solution A primary standard is a reagent that we can use to dispense an accurately known. A solution of accurately known concentration, prepared using standard substances in one of several ways. It typically has a relatively high molar mass that can be accurately measured for comparison. A secondary standard is not that pure and is chemically reactive than primary standards. A. Primary Standard Vs Standard Solution.
From www.slideshare.net
1 Titrations Primary Standard Vs Standard Solution A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations. A standard solution can be prepared directly from a primary standard. A secondary standard is not that pure and is chemically reactive than primary standards. A solution of accurately known concentration, prepared using standard substances in one of several ways.. Primary Standard Vs Standard Solution.
From www.askdifference.com
Primary Standard Solution vs. Secondary Standard Solution — What’s the Primary Standard Vs Standard Solution A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard must have, at least, the following characteristics: It typically has a relatively high molar mass that can be accurately measured for comparison. There are two categories of analytical standards: Primary standards and secondary standards. A standard solution is measured. A primary standard solution. Primary Standard Vs Standard Solution.
From www.differencebetween.com
Difference Between Stock Solution and Standard Solution Compare the Primary Standard Vs Standard Solution A standard solution is measured. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. It must also have a high level of purity, stability, and solubility in water. A standard solution can be prepared. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry / PHC 213 PowerPoint Primary Standard Vs Standard Solution High state of purity, stability in air and in. It must also have a high level of purity, stability, and solubility in water. There are two categories of analytical standards: It typically has a relatively high molar mass that can be accurately measured for comparison. Reagents are chemicals used to cause a chemical reaction with another substance and they are. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Primary Standard Vs Standard Solution Primary standards and secondary standards. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A solution of accurately known concentration, prepared using standard substances in one of several ways. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Standard Solutions PowerPoint Presentation, free download ID Primary Standard Vs Standard Solution A standard solution is measured. There are two categories of analytical standards: A primary standard must have, at least, the following characteristics: A standard solution can be prepared directly from a primary standard. A primary standard solution is a solution with a high purity and less reactivity. High state of purity, stability in air and in. A primary standard is. Primary Standard Vs Standard Solution.
From www.differencebetween.com
Difference Between Primary and Secondary Standard Solution Compare Primary Standard Vs Standard Solution A standard solution is measured. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. It typically has a relatively high molar mass that can be accurately measured for comparison. A secondary standard is not that pure and is chemically reactive than primary standards. A solution of accurately known concentration,. Primary Standard Vs Standard Solution.
From www.chemicals.co.uk
What Is A Standard Solution? The Chemistry Blog Primary Standard Vs Standard Solution It must also have a high level of purity, stability, and solubility in water. A standard solution can be prepared directly from a primary standard. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard solution is a solution with a high purity and less reactivity. A. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Chapter 3 Volumetric Analysis PowerPoint Presentation, free Primary Standard Vs Standard Solution A standard solution is measured. A primary standard must have, at least, the following characteristics: A solution of accurately known concentration, prepared using standard substances in one of several ways. It must also have a high level of purity, stability, and solubility in water. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of. Primary Standard Vs Standard Solution.
From www.youtube.com
Analytical Chemistry Primary standard and secondary standard Primary Standard Vs Standard Solution A secondary standard is not that pure and is chemically reactive than primary standards. A standard solution can be prepared directly from a primary standard. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard is a reagent that we can use to dispense an accurately known.. Primary Standard Vs Standard Solution.
From definitionjks.blogspot.com
Standard Solution Definition Chemistry DEFINITION JKS Primary Standard Vs Standard Solution It must also have a high level of purity, stability, and solubility in water. A solution of accurately known concentration, prepared using standard substances in one of several ways. A standard solution can be prepared directly from a primary standard. A secondary standard is not that pure and is chemically reactive than primary standards. There are two categories of analytical. Primary Standard Vs Standard Solution.
From www.slideshare.net
1 Titrations Primary Standard Vs Standard Solution High state of purity, stability in air and in. A solution of accurately known concentration, prepared using standard substances in one of several ways. A secondary standard is not that pure and is chemically reactive than primary standards. There are two categories of analytical standards: A primary standard solution is a solution with a high purity and less reactivity. It. Primary Standard Vs Standard Solution.
From sciencenotes.org
What Is a Primary Standard in Chemistry? Primary Standard Vs Standard Solution A solution of accurately known concentration, prepared using standard substances in one of several ways. A secondary standard is not that pure and is chemically reactive than primary standards. There are two categories of analytical standards: A standard solution can be prepared directly from a primary standard. A primary standard is a reagent that we can use to dispense an. Primary Standard Vs Standard Solution.
From answerlibbharals.z21.web.core.windows.net
What Is The Primary Standard Primary Standard Vs Standard Solution Primary standards and secondary standards. Reagents are chemicals used to cause a chemical reaction with another substance and they are often used to test for the presence or quantity of specific chemicals in a solution. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard solution is. Primary Standard Vs Standard Solution.
From www.youtube.com
1.3. Primary and Secondary Standards PA1 TPL YouTube Primary Standard Vs Standard Solution A standard solution is measured. A primary standard in chemistry is a pure, easily weighed reagent representative of the number of moles the substance contains. A primary standard is a reagent that we can use to dispense an accurately known. A primary standard solution is a solution with a high purity and less reactivity. It typically has a relatively high. Primary Standard Vs Standard Solution.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Primary Standard Vs Standard Solution A standard solution is measured. A solution of accurately known concentration, prepared using standard substances in one of several ways. A secondary standard is not that pure and is chemically reactive than primary standards. A primary standard must have, at least, the following characteristics: A standard solution can be prepared directly from a primary standard. Reagents are chemicals used to. Primary Standard Vs Standard Solution.