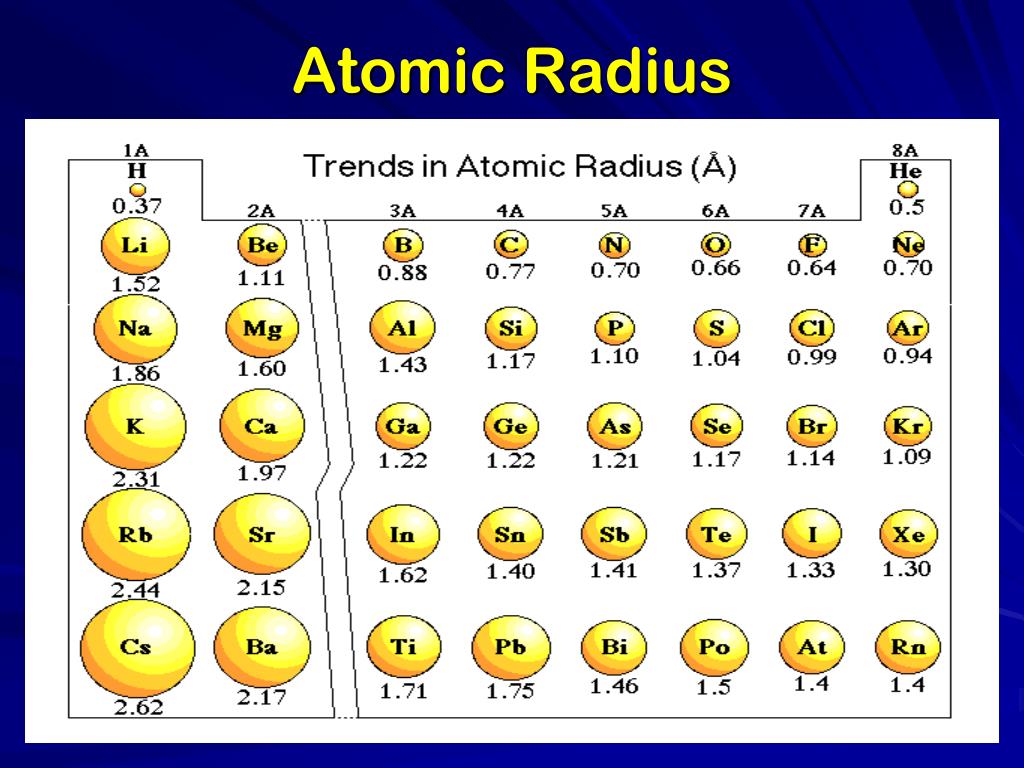
What Is Meant By Atomic Radius . The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. An atom has no rigid. In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. Atomic radius periodic table trends. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. As you begin to move. We define the atomic radius of a chemical element as: Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. However, there is no standard definition for this value. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element.
from www.slideserve.com
The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius is a term used to describe the size of an atom. As you begin to move. However, there is no standard definition for this value. An atom has no rigid. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus.
PPT Periodic Table PowerPoint Presentation, free download ID1951146
What Is Meant By Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. However, there is no standard definition for this value. We define the atomic radius of a chemical element as: In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. As you begin to move. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. An atom has no rigid. Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius periodic table trends.
From ezchem.com
Atomic Radius Trend EZchem What Is Meant By Atomic Radius Atomic radius is a term used to describe the size of an atom. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. In. What Is Meant By Atomic Radius.
From enthu.com
What is the Atomic Radius? EnthuZiastic What Is Meant By Atomic Radius We define the atomic radius of a chemical element as: Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Understanding the atomic radius of elements allows scientists to predict and. What Is Meant By Atomic Radius.
From periodicitychem.weebly.com
Atomic Radius Periodicity Project What Is Meant By Atomic Radius Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius periodic table trends. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. The. What Is Meant By Atomic Radius.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table What Is Meant By Atomic Radius Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The atomic. What Is Meant By Atomic Radius.
From periodictrendsforhonorschemistry.weebly.com
Atomic Radius Trends of the Periodic Table What Is Meant By Atomic Radius However, there is no standard definition for this value. The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. The atomic radius is the size of the atom, typically measured by the. What Is Meant By Atomic Radius.
From www.youtube.com
Atomic Radius and its 4 Types Ionic Radius Covalent Radius What Is Meant By Atomic Radius Atomic radius is a term used to describe the size of an atom. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The mean or typical distance from the centre of. What Is Meant By Atomic Radius.
From www.youtube.com
Atomic Radius Basic Introduction Periodic Table Trends, Chemistry What Is Meant By Atomic Radius We define the atomic radius of a chemical element as: However, there is no standard definition for this value. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the. What Is Meant By Atomic Radius.
From neetlab.com
Atomic Radius Periodic Table NEET Lab What Is Meant By Atomic Radius Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius periodic table trends. An atom has no rigid. We define the atomic radius of a chemical element as: As you begin to move. The atomic radius is the size of the. What Is Meant By Atomic Radius.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps What Is Meant By Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. An atom has no rigid. The atomic radius is the size of the atom, typically measured by the distance from the nucleus. What Is Meant By Atomic Radius.
From www.breakingatom.com
Atomic Radius of Elements What Is Meant By Atomic Radius Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius periodic table trends. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. We define the atomic radius of a chemical element as: Atomic radius is. What Is Meant By Atomic Radius.
From www.slideshare.net
Atomic radius ppt for chem What Is Meant By Atomic Radius Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. We define the atomic radius of a chemical element as: The atomic radius is the size of the atom,. What Is Meant By Atomic Radius.
From www.shutterstock.com
Illustration Chemistry Atomic Radius Measure Size Stock Vector (Royalty What Is Meant By Atomic Radius As you begin to move. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius periodic table trends. The atomic radius may refer to the ionic radius, covalent radius,. What Is Meant By Atomic Radius.
From knordslearning.com
Atomic Radius Trend in Periodic Table (Simple Explanation) What Is Meant By Atomic Radius The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. However, there is no standard definition for this value. An atom has no rigid. We define the atomic radius of a chemical element as: Atomic radius or atomic radii is the total distance from the nucleus of an atom to. What Is Meant By Atomic Radius.
From www.ck12.org
Periodic Trends Atomic Radius CK12 Foundation What Is Meant By Atomic Radius Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. However, there is no standard definition for this value. As you begin to move. Atomic radius, half the distance. What Is Meant By Atomic Radius.
From www.showme.com
Atomic Radius Science ShowMe What Is Meant By Atomic Radius Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. We define the atomic radius of a chemical element as: Atomic radius, half the distance between the nuclei of. What Is Meant By Atomic Radius.
From www.slideserve.com
PPT Atomic radius PowerPoint Presentation, free download ID5235347 What Is Meant By Atomic Radius Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. We define the atomic radius of a chemical element as: In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer. What Is Meant By Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) What Is Meant By Atomic Radius Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. An atom has no rigid. We define the atomic radius of a chemical element as: Atomic radius is. What Is Meant By Atomic Radius.
From www.ck12.org
Atomic Radius Example 2 ( Video ) Chemistry CK12 Foundation What Is Meant By Atomic Radius Atomic radius is a term used to describe the size of an atom. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. As you begin to move. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. The mean or typical. What Is Meant By Atomic Radius.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar What Is Meant By Atomic Radius We define the atomic radius of a chemical element as: The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. As you begin to move. Atomic radius is. What Is Meant By Atomic Radius.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By Atomic Radius Atomic radius periodic table trends. However, there is no standard definition for this value. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. As you begin to. What Is Meant By Atomic Radius.
From www.slideserve.com
PPT PERIODIC TRENDS 1. ATOMIC RADIUS 2. IONIC RADIUS PowerPoint What Is Meant By Atomic Radius As you begin to move. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Atomic radius is a term used. What Is Meant By Atomic Radius.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision What Is Meant By Atomic Radius An atom has no rigid. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Understanding the atomic radius of elements. What Is Meant By Atomic Radius.
From study.com
What is Atomic Radius? Atomic Radius Examples & Periodic Trend What Is Meant By Atomic Radius Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. As you begin to move. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius, half the distance between. What Is Meant By Atomic Radius.
From slidetodoc.com
PERIODIC TRENDS Atomic Radius Atomic radius is the What Is Meant By Atomic Radius The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. However, there is no standard definition for this value. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Understanding the atomic radius of elements allows scientists to predict and explain trends. What Is Meant By Atomic Radius.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry What Is Meant By Atomic Radius As you begin to move. Atomic radius periodic table trends. Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table, such as reactivity and electronegativity. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Covalent radius can be calculated by measuring the distance. What Is Meant By Atomic Radius.
From www.animalia-life.club
Atomic Radius Diagram What Is Meant By Atomic Radius Atomic radius periodic table trends. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius is a term used to describe the size of an atom.. What Is Meant By Atomic Radius.
From www.youtube.com
Atomic Radius Types of atomic radius S Chemistry Live YouTube What Is Meant By Atomic Radius The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Atomic radius periodic table trends. The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. Understanding the atomic radius of elements allows scientists to predict and explain trends in the periodic table,. What Is Meant By Atomic Radius.
From www.teachoo.com
How does atomic radius elements change on going (i) from left to right What Is Meant By Atomic Radius The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is a term used to describe the size of an atom. The atomic radius is the size of. What Is Meant By Atomic Radius.
From animalia-life.club
Atomic Radius Diagram What Is Meant By Atomic Radius In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron.. What Is Meant By Atomic Radius.
From 88guru.com
Atomic RadiusAn Overview 88Guru What Is Meant By Atomic Radius As you begin to move. An atom has no rigid. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Understanding the atomic radius. What Is Meant By Atomic Radius.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table What Is Meant By Atomic Radius The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. We define the atomic radius of a chemical element as: In simpler terms, it can be defined as something similar to the. What Is Meant By Atomic Radius.
From stock.adobe.com
types of atomic radius of a chemical element. Ionic radius vector What Is Meant By Atomic Radius We define the atomic radius of a chemical element as: Atomic radius periodic table trends. In simpler terms, it can be defined as something similar to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. An atom has no rigid. Atomic radius. What Is Meant By Atomic Radius.
From stock.adobe.com
types of atomic radius of a chemical element. Metallic, covalent and What Is Meant By Atomic Radius Atomic radius refers to the size of an atom, typically measured from the center of the nucleus to the outer boundary of the surrounding cloud of electrons. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the. What Is Meant By Atomic Radius.
From in.pinterest.com
Atomic Radius Easy Science Radii, Atom, Flashcards What Is Meant By Atomic Radius Atomic radius is a term used to describe the size of an atom. We define the atomic radius of a chemical element as: The mean or typical distance from the centre of the nucleus to the boundary of the surrounding shells of electrons. Atomic radius periodic table trends. Atomic radius, half the distance between the nuclei of identical neighbouring atoms. What Is Meant By Atomic Radius.
From eduinput.com
Atomic radiusDefinition, examples, variation in periodic table What Is Meant By Atomic Radius The atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals radius. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. However, there is no standard definition for this value. The mean or typical distance from the centre of the nucleus to the boundary. What Is Meant By Atomic Radius.