Medical Device Over Labeling . In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. A review of the performance of a medical device based upon data already available, scientific literature and,. Label (as set out in the act): Premarket notifications (510(k)), establishment registration, device listing,. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Overview of regulations for medical devices:
from mavink.com
In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. A review of the performance of a medical device based upon data already available, scientific literature and,. Overview of regulations for medical devices: Premarket notifications (510(k)), establishment registration, device listing,. Label (as set out in the act): Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its.
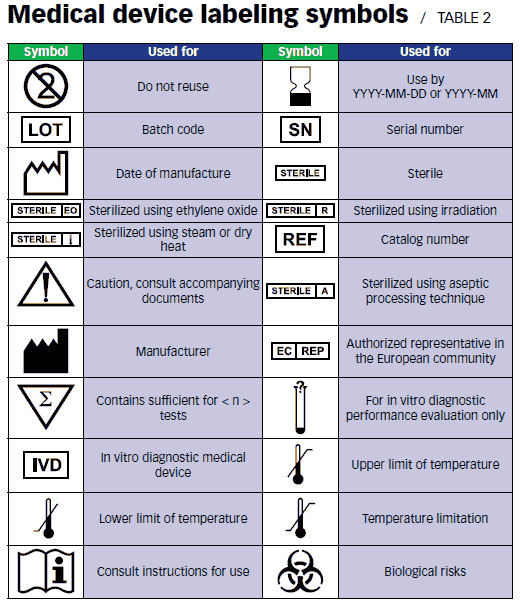
Medical Device Labeling Symbols
Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Label (as set out in the act): A review of the performance of a medical device based upon data already available, scientific literature and,. Premarket notifications (510(k)), establishment registration, device listing,. Overview of regulations for medical devices: In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Over Labeling Label (as set out in the act): Overview of regulations for medical devices: A review of the performance of a medical device based upon data already available, scientific literature and,. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. The purpose of this imdrf guidance is to provide globally. Medical Device Over Labeling.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Over Labeling A review of the performance of a medical device based upon data already available, scientific literature and,. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Premarket notifications (510(k)), establishment registration, device listing,. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical. Medical Device Over Labeling.
From www.medicaldevice-network.com
Understanding updates on medical device labelling Medical Device Network Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. In relation to a health product or an active ingredient, means any written, printed or graphic representation that.. Medical Device Over Labeling.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Medical Device Over Labeling In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic.. Medical Device Over Labeling.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Over Labeling Premarket notifications (510(k)), establishment registration, device listing,. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. The purpose of this imdrf guidance is to provide globally harmonized. Medical Device Over Labeling.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Over Labeling In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling.. Medical Device Over Labeling.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Over Labeling Label (as set out in the act): In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Premarket notifications (510(k)), establishment registration, device listing,. The health services authority (hsa), singapore’s regulatory agency. Medical Device Over Labeling.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Medical Device Over Labeling Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. Overview of regulations for medical devices: A review of the performance of a medical device based upon data already available, scientific literature and,. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a. Medical Device Over Labeling.
From mungfali.com
Medical Device Labeling Symbols Medical Device Over Labeling Overview of regulations for medical devices: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. In relation to a health product or an active ingredient, means any. Medical Device Over Labeling.
From mavink.com
Medical Device Labeling Symbols Medical Device Over Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. A review of the performance of a medical device based upon data already available, scientific literature and,. Label (as set out in the act): Premarket notifications (510(k)), establishment registration, device listing,. The health services authority (hsa), singapore’s regulatory agency in. Medical Device Over Labeling.
From www.londontranslations.co.uk
Medical device labelling What is it & what are the requirements? Medical Device Over Labeling Overview of regulations for medical devices: Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. Premarket notifications (510(k)), establishment registration, device listing,. Label (as set out in the act): The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated. Medical Device Over Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Premarket notifications (510(k)), establishment registration, device listing,. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Label (as set out in the act): A review of the. Medical Device Over Labeling.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Over Labeling In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Overview of regulations for medical devices: A review of the performance of a medical device based upon data already available, scientific literature. Medical Device Over Labeling.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Over Labeling A review of the performance of a medical device based upon data already available, scientific literature and,. Overview of regulations for medical devices: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a. Medical Device Over Labeling.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Over Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Label (as set out in the act): The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has. Medical Device Over Labeling.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Over Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling.. Medical Device Over Labeling.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Medical Device Over Labeling Premarket notifications (510(k)), establishment registration, device listing,. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Label (as set out in the act): The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. A review of the performance of. Medical Device Over Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Over Labeling Overview of regulations for medical devices: Label (as set out in the act): The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. A review of the performance of a medical device based upon data already available, scientific literature and,. The purpose of this imdrf guidance is to. Medical Device Over Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Over Labeling The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. A review of the performance of a medical device based upon data already available, scientific literature and,. Under both regulations the label. Medical Device Over Labeling.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Over Labeling Overview of regulations for medical devices: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Label (as set out in the act): A review of the performance of a medical device based upon data already available, scientific literature and,. Under both regulations the label (or labeling) includes all written,. Medical Device Over Labeling.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Medical Device Over Labeling Overview of regulations for medical devices: Label (as set out in the act): In relation to a health product or an active ingredient, means any written, printed or graphic representation that. A review of the performance of a medical device based upon data already available, scientific literature and,. Under both regulations the label (or labeling) includes all written, graphic, or. Medical Device Over Labeling.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Over Labeling Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. Overview of regulations for medical devices: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. A review of the performance of a medical device based upon data already available,. Medical Device Over Labeling.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Medical Device Over Labeling Label (as set out in the act): Overview of regulations for medical devices: The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. A review of the performance. Medical Device Over Labeling.
From andamanmed.com
MDA/GD/0026 Labelling of Medical Devices Andaman Medical Medical Device Over Labeling Overview of regulations for medical devices: Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. A review of the performance of a medical device based upon data already available, scientific literature and,. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including. Medical Device Over Labeling.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Overview of regulations for medical devices: Label (as set out in the act): In relation to a health product or an active ingredient, means any written, printed or graphic representation that. The purpose of this imdrf guidance is. Medical Device Over Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Over Labeling Label (as set out in the act): Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. Premarket notifications (510(k)), establishment registration, device listing,. Overview of regulations for medical devices: A review of the performance of a medical device based upon data already available, scientific literature and,. In relation to. Medical Device Over Labeling.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Medical Device Over Labeling Label (as set out in the act): The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. A review of the performance of a medical device based upon data already available, scientific literature and,. In relation to a health product or an active ingredient, means any written, printed. Medical Device Over Labeling.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Over Labeling Premarket notifications (510(k)), establishment registration, device listing,. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Overview of regulations for medical devices: Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. The purpose of this imdrf guidance is to provide. Medical Device Over Labeling.
From www.dreamstime.com
Medical Device Labeling Symbols Stock Illustrations 3 Medical Device Medical Device Over Labeling Overview of regulations for medical devices: The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to. Medical Device Over Labeling.
From www.scribd.com
Requirements For Labelling of Medical Devices Mda PDF Medical Medical Device Over Labeling Label (as set out in the act): The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Under both regulations the label (or labeling) includes all written, graphic,. Medical Device Over Labeling.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Medical Device Over Labeling Premarket notifications (510(k)), establishment registration, device listing,. Overview of regulations for medical devices: The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. A review of the performance of a medical device based upon data already available, scientific literature and,. Label (as set out in the act): The. Medical Device Over Labeling.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Over Labeling Label (as set out in the act): The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices, including in vitro diagnostic. Under both regulations the label (or labeling) includes all written, graphic, or printed matter affixed to a medical device, its. Overview of regulations for medical devices: The health services authority (hsa), singapore’s regulatory. Medical Device Over Labeling.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Over Labeling Premarket notifications (510(k)), establishment registration, device listing,. The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Under both regulations the label (or labeling) includes all written, graphic, or printed. Medical Device Over Labeling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. Premarket notifications (510(k)), establishment registration, device listing,. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Label (as set out in the act): Under both regulations the label (or. Medical Device Over Labeling.
From www.schlafenderhase.com
ELabelling Practical Next Steps for Medical Device Manufacturers Medical Device Over Labeling The health services authority (hsa), singapore’s regulatory agency in the sphere of healthcare products, has published a guidance document dedicated to labeling. In relation to a health product or an active ingredient, means any written, printed or graphic representation that. Premarket notifications (510(k)), establishment registration, device listing,. Label (as set out in the act): Overview of regulations for medical devices:. Medical Device Over Labeling.